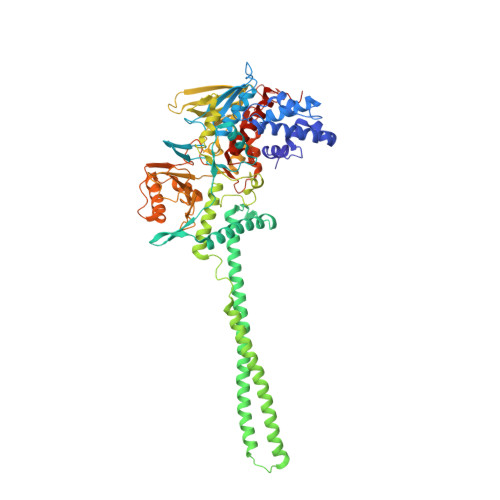
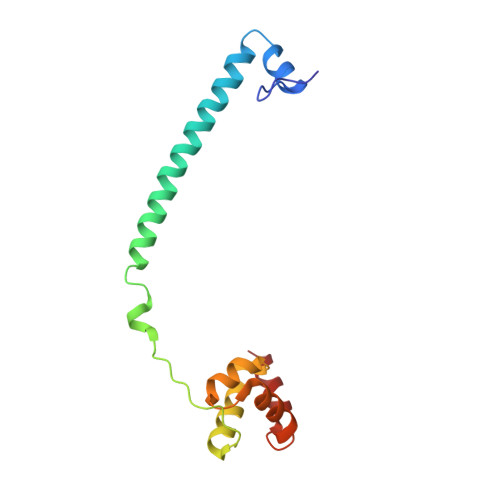
Phosphorylation of Neuronal Lysine-Specific Demethylase 1Lsd1/Kdm1A Impairs Transcriptional Repression by Regulating Interaction with Corest and Histone Deacetylases Hdac1/2.
Toffolo, E., Rusconi, F., Paganini, L., Tortorici, M., Pilotto, S., Heise, C., Verpelli, C., Tedeschi, G., Maffioli, E., Sala, C., Mattevi, A., Battaglioli, E.(2014) J Neurochem 128: 616
- PubMed: 24111946
- DOI: https://doi.org/10.1111/jnc.12457
- Primary Citation of Related Structures:
4BAY - PubMed Abstract:
Epigenetic mechanisms play important roles in brain development, orchestrating proliferation, differentiation, and morphogenesis. Lysine-Specific Demethylase 1 (LSD1 also known as KDM1A and AOF2) is a histone modifier involved in transcriptional repression, forming a stable core complex with the corepressors corepressor of REST (CoREST) and histone deacetylases (HDAC1/2). Importantly, in the mammalian CNS, neuronal LSD1-8a, an alternative splicing isoform of LSD1 including the mini-exon E8a, sets alongside LSD1 and is capable of enhancing neurite growth and morphogenesis. Here, we describe that the morphogenic properties of neuronal LSD1-8a require switching off repressive activity and this negative modulation is mediated in vivo by phosphorylation of the Thr369b residue coded by exon E8a. Three-dimensional crystal structure analysis using a phospho-mimetic mutant (Thr369bAsp), indicate that phosphorylation affects the residues surrounding the exon E8a-coded amino acids, causing a local conformational change. We suggest that phosphorylation, without affecting demethylase activity, causes in neurons CoREST and HDAC1/2 corepressors detachment from LSD1-8a and impairs neuronal LSD1-8a repressive activity. In neurons, Thr369b phosphorylation is required for morphogenic activity, converting neuronal LSD1-8a in a dominant-negative isoform, challenging LSD1-mediated transcriptional repression on target genes.
- Department of Medical Biotechnology and Translational Medicine, University of Milan, Milan, Italy.
Organizational Affiliation: