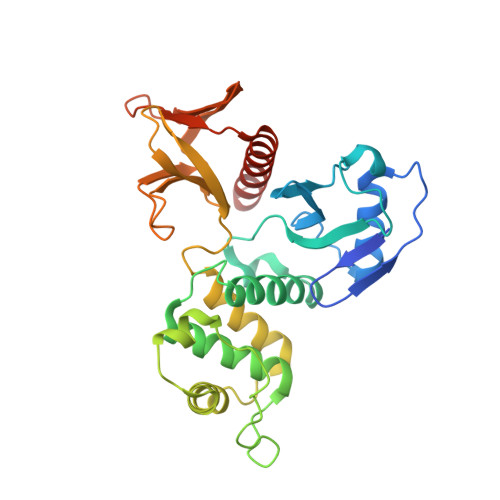
Structural basis of DDB1-and-Cullin 4-associated Factor 1 (DCAF1) recognition by merlin/NF2 and its implication in tumorigenesis by CD44-mediated inhibition of merlin suppression of DCAF1 function.
Mori, T., Gotoh, S., Shirakawa, M., Hakoshima, T.(2014) Genes Cells 19: 603-619
- PubMed: 24912773
- DOI: https://doi.org/10.1111/gtc.12161
- Primary Citation of Related Structures:
3WA0 - PubMed Abstract:
Merlin, a tumor suppressor encoded by the neurofibromatosis type 2 gene, has been shown to suppress tumorigenesis by inhibiting the Cullin 4-RING E3 ubiquitin ligase CRL4(DCAF) (1) in the nucleus. This inhibition is mediated by direct binding of merlin to DDB1-and-Cullin 4-associated Factor 1 (DCAF1), yet the binding mode of merlin to DCAF1 is not well defined. Here, we report structural and biophysical studies of the merlin binding to DCAF1 and its interference with CD44 binding. The crystal structure of the merlin FERM domain bound to the DCAF1 C-terminal acidic tail reveals that the hydrophobic IILXLN motif located at the C-terminal end of DCAF1 binds subdomain C of the FERM domain by forming a β-strand. The binding site and mode resemble that of merlin binding to the CD44 cytoplasmic tail. Competition binding assay showed that CD44 and DCAF1 compete for binding to the merlin FERM domain in solution. The CD44 cytoplasmic tail is known to be cleaved for nuclear translocation by regulated intra-membrane proteolysis (RIP). Our structure implies that, in the nucleus, the CD44 cytoplasmic tail cleaved by RIP could release DCAF1 from merlin by competing for binding to the merlin FERM domain, which results in the inhibition of merlin-mediated suppression of tumorigenesis.
- Structural Biology Laboratory, Nara Institute of Science and Technology, 8916-5, Takayama, Ikoma, Nara, 630-0192, Japan.
Organizational Affiliation: