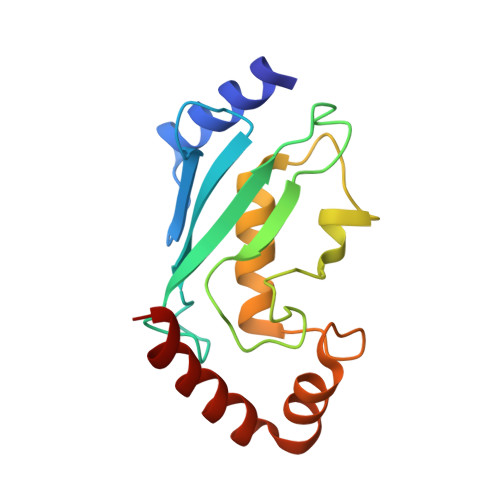
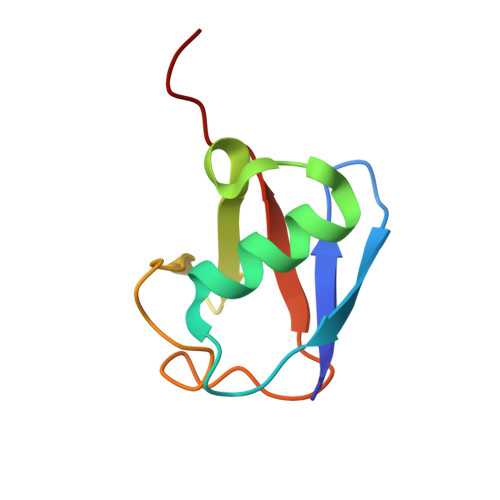
Structural insights into the conformation and oligomerization of E2~ubiquitin conjugates.
Page, R.C., Pruneda, J.N., Amick, J., Klevit, R.E., Misra, S.(2012) Biochemistry 51: 4175-4187
- PubMed: 22551455
- DOI: https://doi.org/10.1021/bi300058m
- Primary Citation of Related Structures:
3UGB - PubMed Abstract:
Post-translational modification of proteins by ubiquitin (Ub) regulates a host of cellular processes, including protein quality control, DNA repair, endocytosis, and cellular signaling. In the ubiquitination cascade, a thioester-linked conjugate between the C-terminus of Ub and the active site cysteine of a ubiquitin-conjugating enzyme (E2) is formed. The E2~Ub conjugate interacts with a ubiquitin ligase (E3) to transfer Ub to a lysine residue on a target protein. The flexibly linked E2~Ub conjugates have been shown to form a range of structures in solution. In addition, select E2~Ub conjugates oligomerize through a noncovalent "backside" interaction between Ub and E2 components of different conjugates. Additional studies are needed to bridge the gap between the dynamic monomeric conjugates, E2~Ub oligomers, and the mechanisms of ubiquitination. We present a new 2.35 Å crystal structure of an oligomeric UbcH5c~Ub conjugate. The conjugate forms a staggered linear oligomer that differs substantially from the "infinite spiral" helical arrangement of the only previously reported structure of an oligomeric conjugate. Our structure also differs in intraconjugate conformation from other structurally characterized conjugates. Despite these differences, we find that the backside interaction mode is conserved in different conjugate oligomers and is independent of intraconjugate relative E2-Ub orientations. We delineate a common intraconjugate E2-binding surface on Ub. In addition, we demonstrate that an E3 CHIP (carboxyl terminus of Hsp70 interacting protein) interacts directly with UbcH5c~Ub oligomers, not only with conjugate monomers. These results provide insights into the conformational diversity of E2~Ub conjugates and conjugate oligomers, and into their compatibility and interactions with E3s, which have important consequences for the ubiquitination process.
- Department of Molecular Cardiology, Lerner Research Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA. pager2@ccf.org
Organizational Affiliation: