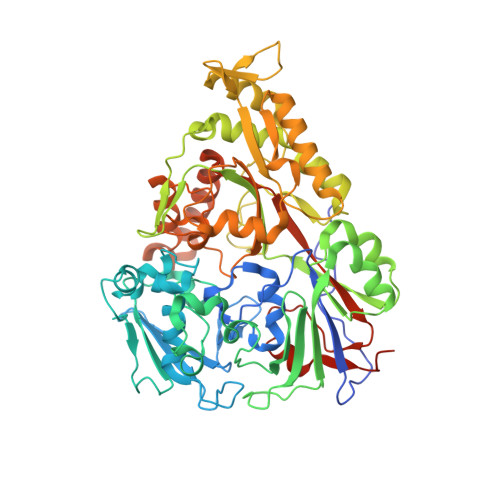
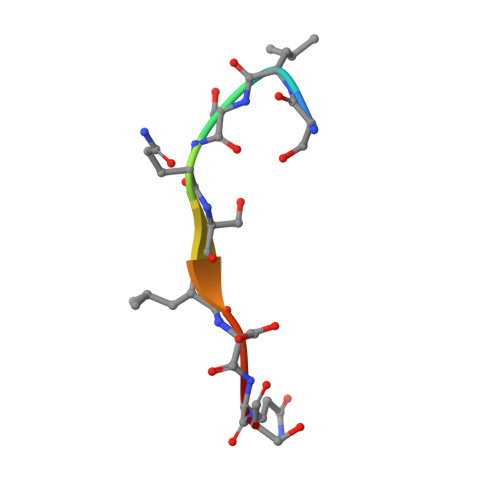
Importance of a Hydrophobic Pocket for Peptide Binding in Lactococcal OppA.
Berntsson, R.P., Thunnissen, A.M., Poolman, B., Slotboom, D.J.(2011) J Bacteriol 193: 4254-4256
- PubMed: 21665971
- DOI: https://doi.org/10.1128/JB.00447-11
- Primary Citation of Related Structures:
3RYA, 3RYB - PubMed Abstract:
Lactococcal oligopeptide-binding protein A (OppA) binds peptides with widely varied lengths and sequences. We previously hypothesized that a hydrophobic pocket in OppA preferentially binds a hydrophobic peptide side chain and thus determines its binding register. Two crystal structures of OppA with different nonapeptides now indeed show binding in different registers.
- Biochemistry Department, Groningen Biomolecular Sciences and Biotechnology Center, NPC & Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands.
Organizational Affiliation: