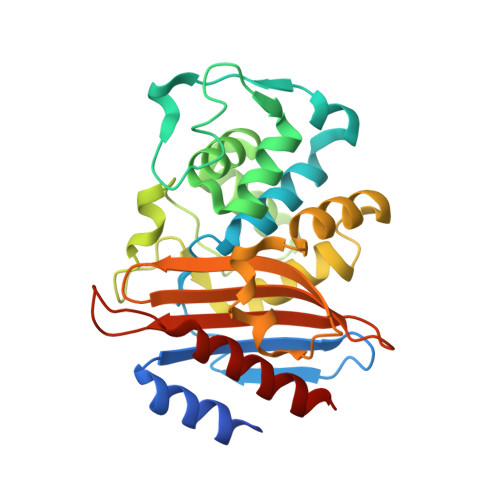
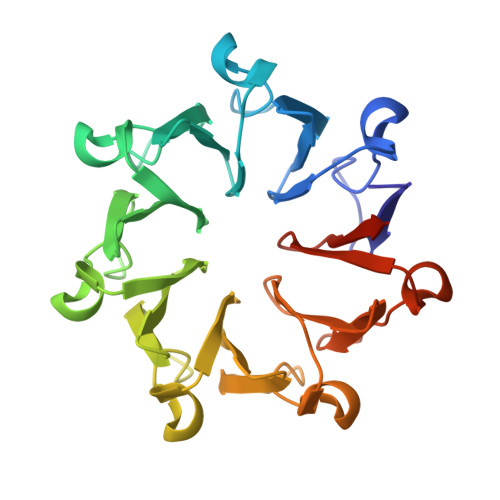
Analysis of the binding forces driving the tight interactions between beta-lactamase inhibitory protein-II (BLIP-II) and class A beta-lactamases.
Brown, N.G., Chow, D.C., Sankaran, B., Zwart, P., Prasad, B.V., Palzkill, T.(2011) J Biological Chem 286: 32723-32735
- PubMed: 21775426
- DOI: https://doi.org/10.1074/jbc.M111.265058
- Primary Citation of Related Structures:
3QHY, 3QI0 - PubMed Abstract:
β-Lactamases hydrolyze β-lactam antibiotics to provide drug resistance to bacteria. β-Lactamase inhibitory protein-II (BLIP-II) is a potent proteinaceous inhibitor that exhibits low picomolar affinity for class A β-lactamases. This study examines the driving forces for binding between BLIP-II and β-lactamases using a combination of presteady state kinetics, isothermal titration calorimetry, and x-ray crystallography. The measured dissociation rate constants for BLIP-II and various β-lactamases ranged from 10(-4) to 10(-7) s(-1) and are comparable with those found in some of the tightest known protein-protein interactions. The crystal structures of BLIP-II alone and in complex with Bacillus anthracis Bla1 β-lactamase revealed no significant side-chain movement in BLIP-II in the complex versus the monomer. The structural rigidity of BLIP-II minimizes the loss of the entropy upon complex formation and, as indicated by thermodynamics experiments, may be a key determinant of the observed potent inhibition of β-lactamases.
- Department of Pharmacology, Baylor College of Medicine, Houston, Texas 77030, USA.
Organizational Affiliation: