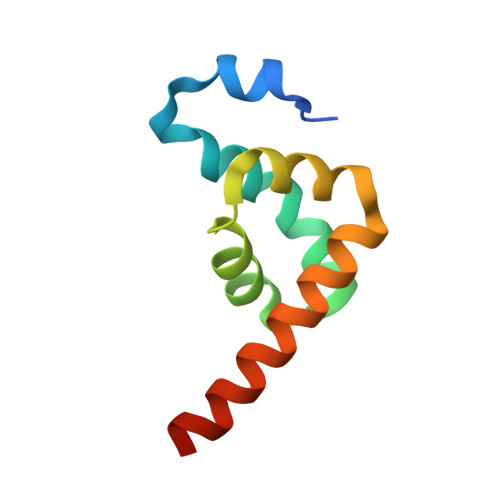
La-Related Protein 4 Binds Poly(A), Interacts with the Poly(A)-Binding Protein MLLE Domain via a Variant PAM2w Motif, and Can Promote mRNA Stability.
Yang, R., Gaidamakov, S.A., Xie, J., Lee, J., Martino, L., Kozlov, G., Crawford, A.K., Russo, A.N., Conte, M.R., Gehring, K., Maraia, R.J.(2011) Mol Cell Biol 31: 542-556
- PubMed: 21098120
- DOI: https://doi.org/10.1128/MCB.01162-10
- Primary Citation of Related Structures:
3PKN - PubMed Abstract:
The conserved RNA binding protein La recognizes UUU-3'OH on its small nuclear RNA ligands and stabilizes them against 3'-end-mediated decay. We report that newly described La-related protein 4 (LARP4) is a factor that can bind poly(A) RNA and interact with poly(A) binding protein (PABP). Yeast two-hybrid analysis and reciprocal immunoprecipitations (IPs) from HeLa cells revealed that LARP4 interacts with RACK1, a 40S ribosome- and mRNA-associated protein. LARP4 cosediments with 40S ribosome subunits and polyribosomes, and its knockdown decreases translation. Mutagenesis of the RNA binding or PABP interaction motifs decrease LARP4 association with polysomes. Several translation and mRNA metabolism-related proteins use a PAM2 sequence containing a critical invariant phenylalanine to make direct contact with the MLLE domain of PABP, and their competition for the MLLE is thought to regulate mRNA homeostasis. Unlike all ∼150 previously analyzed PAM2 sequences, LARP4 contains a variant PAM2 (PAM2w) with tryptophan in place of the phenylalanine. Binding and nuclear magnetic resonance (NMR) studies have shown that a peptide representing LARP4 PAM2w interacts with the MLLE of PABP within the affinity range measured for other PAM2 motif peptides. A cocrystal of PABC bound to LARP4 PAM2w shows tryptophan in the pocket in PABC-MLLE otherwise occupied by phenylalanine. We present evidence that LARP4 expression stimulates luciferase reporter activity by promoting mRNA stability, as shown by mRNA decay analysis of luciferase and cellular mRNAs. We propose that LARP4 activity is integrated with other PAM2 protein activities by PABP as part of mRNA homeostasis.
- 31 Center Drive, Bldg. 31, Room 2A25, Bethesda, MD 20892-2426, USA.
Organizational Affiliation: