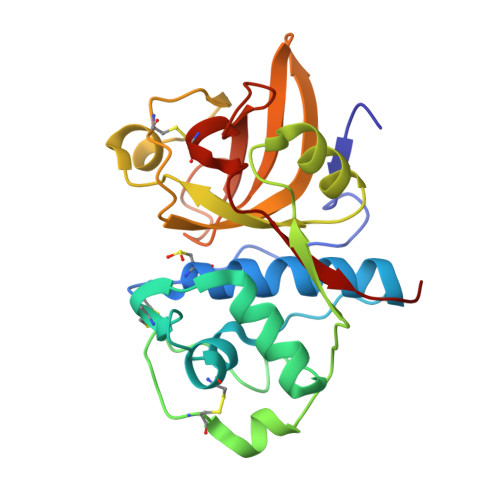
Structural analysis of actinidin and a comparison of cadmium and sulfur anomalous signals from actinidin crystals measured using in-house copper- and chromium-anode X-ray sources
Yogavel, M., Nithya, N., Suzuki, A., Sugiyama, Y., Yamane, T., Velmurugan, D., Sharma, A.(2010) Acta Crystallogr D Biol Crystallogr 66: 1323-1333
- PubMed: 21123873
- DOI: https://doi.org/10.1107/S0907444910040394
- Primary Citation of Related Structures:
3P5U, 3P5V, 3P5W, 3P5X - PubMed Abstract:
The structure of the 24 kDa cysteine protease saru-actinidin from the fruit of Actinidia arguta Planch. (sarunashi) was determined by the cadmium/sulfur-SAD method with X-ray diffraction data collected using in-house Cu Kα and Cr Kα radiation. The anomalous scatterers included nine sulfurs and several cadmium ions from the crystallization solution. The high quality of the diffraction data, the use of chromium-anode X-ray radiation and the substantial anomalous signal allowed structure determination and automated model building despite both a low solvent content (<40%) and low data multiplicity. The amino-acid sequence of saru-actinidin was deduced from the cDNA and was modified based on experimental electron-density maps at 1.5 Å resolution. The active site of saru-actinidin is occupied by a cadmium ion and the active-site cysteine is found to be in an unmodified, cysteine sulfenic acid or cysteine sulfinic acid form. The cadmium sites, coordination geometries and polygonal water structures on the protein surface have also been extensively analyzed. An analysis and comparison of the sulfur/cadmium anomalous signals at the Cu Kα and Cr Kα wavelengths was carried out. It is proposed that the inclusion of cadmium salts in crystallization solutions coupled with chromium-anode radiation can provide a convenient route for structure determination.
- Structural and Computational Biology Group, International Centre for Genetic Engineering and Biotechnology, Aruna Asaf Ali Road, New Delhi 110067, India.
Organizational Affiliation: