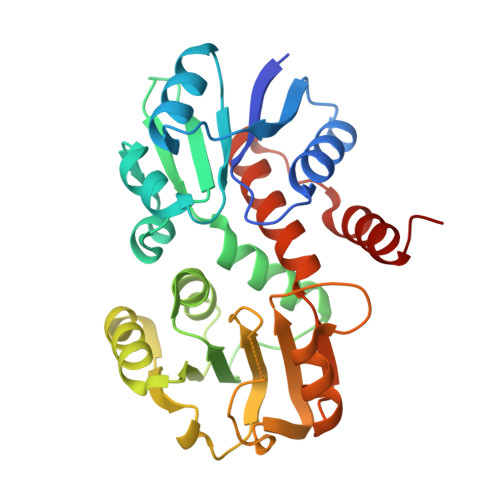
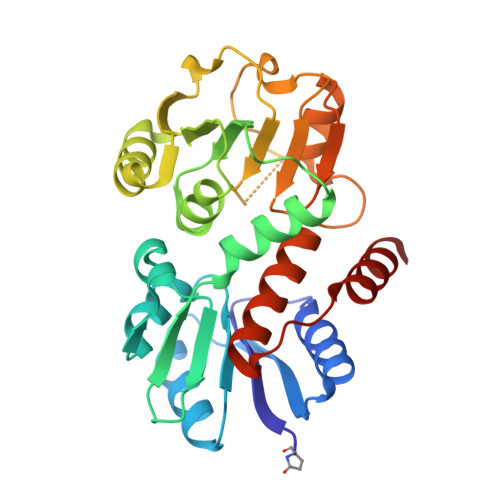
1.45 Angstrom Resolution Crystal Structure of Shikimate 5-Dehydrogenase (aroE) from Vibrio cholerae.
Minasov, G., Light, S.H., Shuvalova, L., Papazisi, L., Anderson, W.F., Center for Structural Genomics of Infectious Diseases (CSGID)To be published.