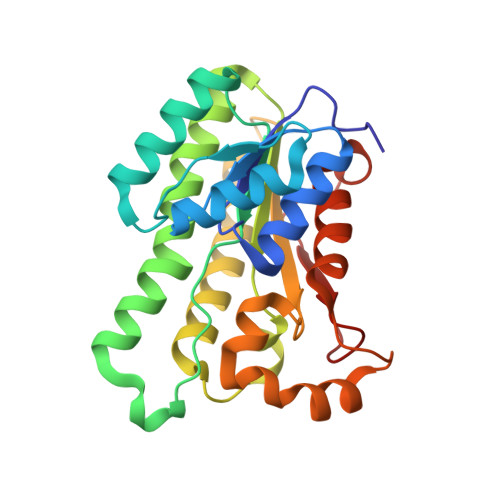
The crystal structure of SDR-type pyridoxal 4-dehydrogenase of Mesorhizobium loti
Chu, H.N., Kobayashi, J., Mikami, B., Yagi, T.(2011) Biosci Biotechnol Biochem 75: 388-390
- PubMed: 21307574
- DOI: https://doi.org/10.1271/bbb.100748
- Primary Citation of Related Structures:
3NUG - PubMed Abstract:
Pyridoxal 4-dehydrogenase catalyzes the irreversible oxidation of pyridoxal to 4-pyridoxolactone and is involved in degradation pathway I of pyridoxine, a vitamin B(6) compound. Its crystal structure was elucidated for the first time. Molecular replacement with (S)-1-phenylthanol dehydrogenase (PDB code 2EW8) was adopted to determine the tertiary structure of the NAD(+)-bound enzyme.
- Faculty of Agriculture and Agricultural Science Program, Graduate School of Integral Arts and Science, Kochi University, Nankoku, Kochi, Japan.
Organizational Affiliation: