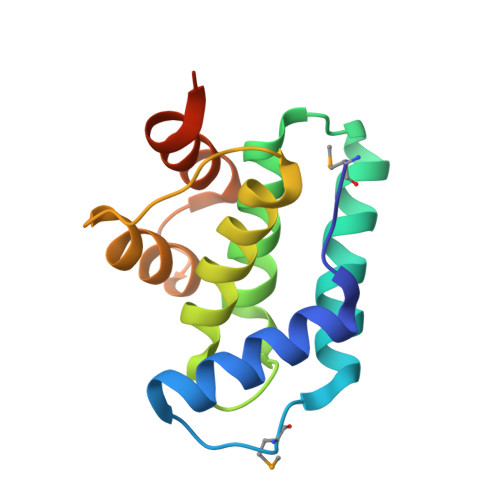
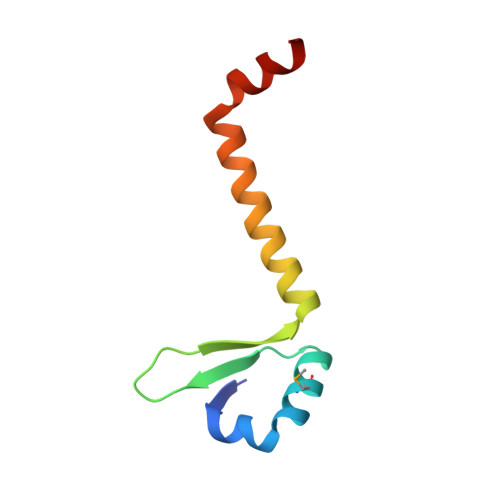
Crystal Structures of Phd-Doc, HigA, and YeeU Establish Multiple Evolutionary Links between Microbial Growth-Regulating Toxin-Antitoxin Systems.
Arbing, M.A., Handelman, S.K., Kuzin, A.P., Verdon, G., Wang, C., Su, M., Rothenbacher, F.P., Abashidze, M., Liu, M., Hurley, J.M., Xiao, R., Acton, T., Inouye, M., Montelione, G.T., Woychik, N.A., Hunt, J.F.(2010) Structure 18: 996-1010
- PubMed: 20696400
- DOI: https://doi.org/10.1016/j.str.2010.04.018
- Primary Citation of Related Structures:
2H28, 2ICT, 2INW, 3KH2 - PubMed Abstract:
Bacterial toxin-antitoxin (TA) systems serve a variety of physiological functions including regulation of cell growth and maintenance of foreign genetic elements. Sequence analyses suggest that TA families are linked by complex evolutionary relationships reflecting likely swapping of functional domains between different TA families. Our crystal structures of Phd-Doc from bacteriophage P1, the HigA antitoxin from Escherichia coli CFT073, and YeeU of the YeeUWV systems from E. coli K12 and Shigella flexneri confirm this inference and reveal additional, unanticipated structural relationships. The growth-regulating Doc toxin exhibits structural similarity to secreted virulence factors that are toxic for eukaryotic target cells. The Phd antitoxin possesses the same fold as both the YefM and NE2111 antitoxins that inhibit structurally unrelated toxins. YeeU, which has an antitoxin-like activity that represses toxin expression, is structurally similar to the ribosome-interacting toxins YoeB and RelE. These observations suggest extensive functional exchanges have occurred between TA systems during bacterial evolution.
- Department of Biological Sciences, Columbia University, 702 Fairchild Center, MC2434, New York, NY 10027, USA.
Organizational Affiliation: