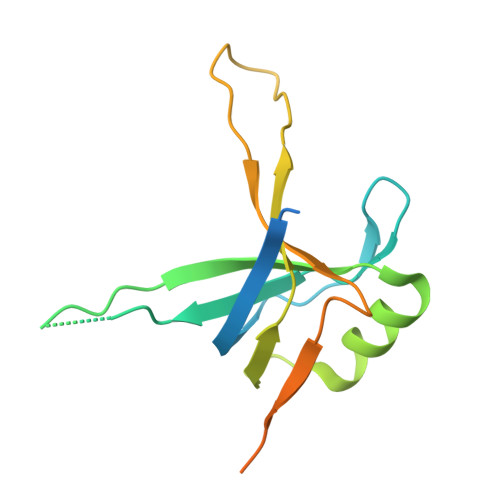
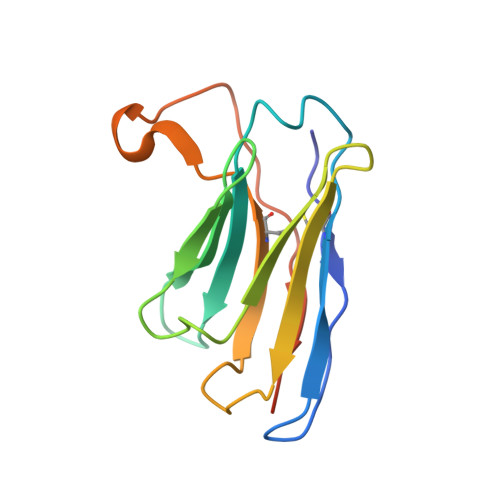
Structures of a key interaction protein from the Trypanosoma brucei editosome in complex with single domain antibodies.
Wu, M., Park, Y.J., Pardon, E., Turley, S., Hayhurst, A., Deng, J., Steyaert, J., Hol, W.G.(2011) J Struct Biol 174: 124-136
- PubMed: 20969962
- DOI: https://doi.org/10.1016/j.jsb.2010.10.007
- Primary Citation of Related Structures:
3K7U, 3K80, 3K81 - PubMed Abstract:
Several major global diseases are caused by single-cell parasites called trypanosomatids. These organisms exhibit many unusual features including a unique and essential U-insertion/deletion RNA editing process in their single mitochondrion. Many key RNA editing steps occur in ∼20S editosomes, which have a core of 12 proteins. Among these, the "interaction protein" KREPA6 performs a central role in maintaining the integrity of the editosome core and also binds to ssRNA. The use of llama single domain antibodies (VHH domains) accelerated crystal growth of KREPA6 from Trypanosoma brucei dramatically. All three structures obtained are heterotetramers with a KREPA6 dimer in the center, and one VHH domain bound to each KREPA6 subunit. Two of the resultant heterotetramers use complementarity determining region 2 (CDR2) and framework residues to form a parallel pair of beta strands with KREPA6 - a mode of interaction not seen before in VHH domain-protein antigen complexes. The third type of VHH domain binds in a totally different manner to KREPA6. Intriguingly, while KREPA6 forms tetramers in solution adding either one of the three VHH domains results in the formation of a heterotetramer in solution, in perfect agreement with the crystal structures. Biochemical solution studies indicate that the C-terminal tail of KREPA6 is involved in the dimerization of KREPA6 dimers to form tetramers. The implications of these crystallographic and solution studies for possible modes of interaction of KREPA6 with its many binding partners in the editosome are discussed.
- Biomolecular Structure Center, Department of Biochemistry, School of Medicine, University of Washington, Seattle, WA 98195, USA.
Organizational Affiliation: