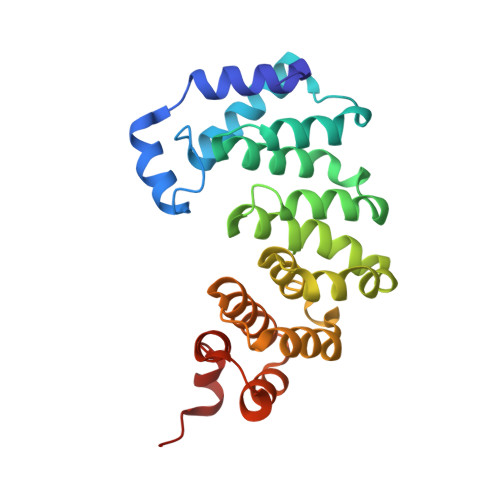
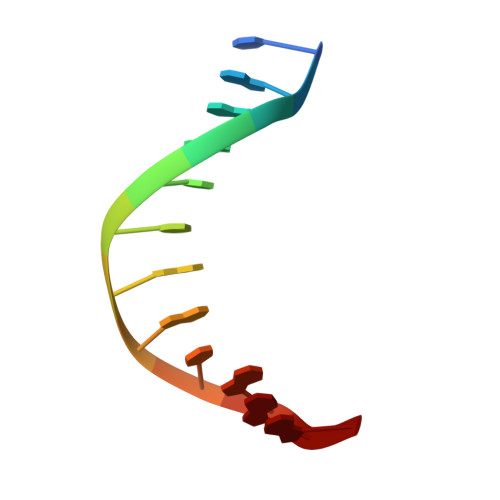
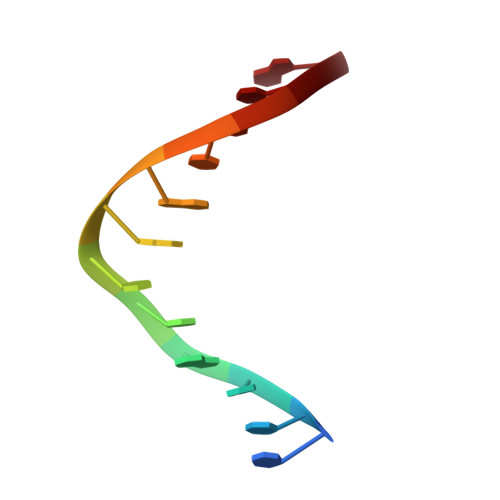
An unprecedented nucleic acid capture mechanism for excision of DNA damage.
Rubinson, E.H., Gowda, A.S., Spratt, T.E., Gold, B., Eichman, B.F.(2010) Nature 468: 406-411
- PubMed: 20927102
- DOI: https://doi.org/10.1038/nature09428
- Primary Citation of Related Structures:
3JX7, 3JXY, 3JXZ, 3JY1 - PubMed Abstract:
DNA glycosylases that remove alkylated and deaminated purine nucleobases are essential DNA repair enzymes that protect the genome, and at the same time confound cancer alkylation therapy, by excising cytotoxic N3-methyladenine bases formed by DNA-targeting anticancer compounds. The basis for glycosylase specificity towards N3- and N7-alkylpurines is believed to result from intrinsic instability of the modified bases and not from direct enzyme functional group chemistry. Here we present crystal structures of the recently discovered Bacillus cereus AlkD glycosylase in complex with DNAs containing alkylated, mismatched and abasic nucleotides. Unlike other glycosylases, AlkD captures the extrahelical lesion in a solvent-exposed orientation, providing an illustration for how hydrolysis of N3- and N7-alkylated bases may be facilitated by increased lifetime out of the DNA helix. The structures and supporting biochemical analysis of base flipping and catalysis reveal how the HEAT repeats of AlkD distort the DNA backbone to detect non-Watson-Crick base pairs without duplex intercalation.
- Department of Biological Sciences and Center for Structural Biology, Vanderbilt University, Nashville, Tennessee 37232, USA.
Organizational Affiliation: