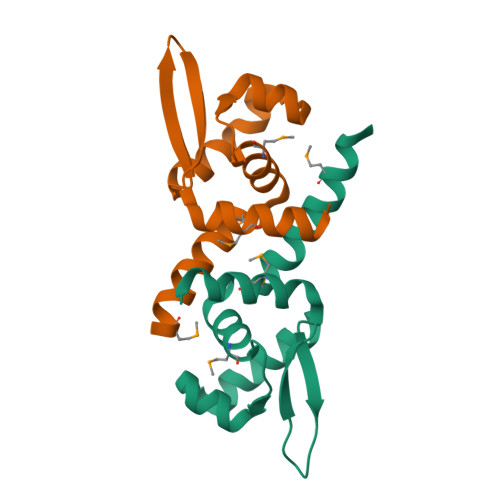
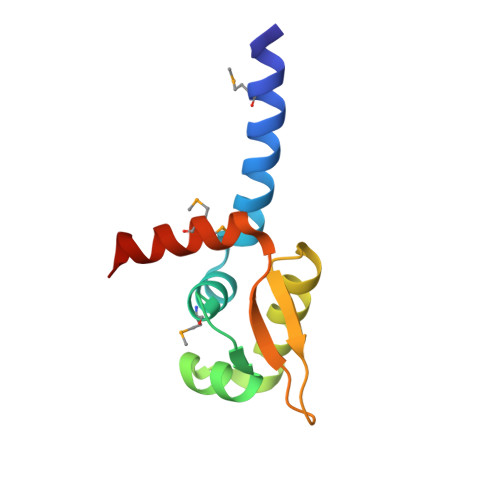
Crystal structure of the transcriptional activator HlyU from Vibrio vulnificus CMCP6.
Nishi, K., Lee, H.J., Park, S.Y., Bae, S.J., Lee, S.E., Adams, P.D., Rhee, J.H., Kim, J.S.(2010) FEBS Lett
- PubMed: 20178784
- DOI: https://doi.org/10.1016/j.febslet.2010.02.052
- Primary Citation of Related Structures:
3JTH - PubMed Abstract:
HlyU is a transcription factor of the ArsR/SmtB family and activates the expression of the pathogenic Vibrio vulnificus RTX toxin. In contrast to the other metal-responding ArsR/SmtB proteins, HlyU does not sense metal ions. To provide its structural information, we elucidated the crystal structure of HlyU from V. vulnificus CMCP6 (HlyU_Vv). The monomeric HlyU_Vv architecture of five alpha-helices and two beta-strands, some of which constitute a typical DNA-binding winged helix-turn-helix (wHTH) motif, is very similar to that of other transcription regulators. Nonetheless, the homo-dimeric HlyU_Vv structure shows several different, three-dimensional features in the spatial position and the detailed dimeric interaction, which were not observed in the modeling study based on the same protein family and sequence similarity.
Organizational Affiliation:
Department of Chemistry, Chonnam National University, Gwangju, Republic of Korea.