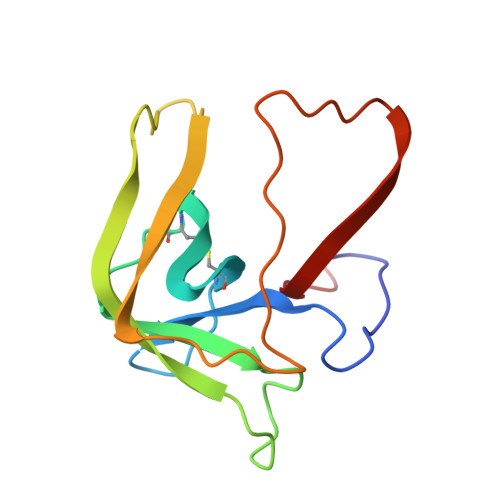
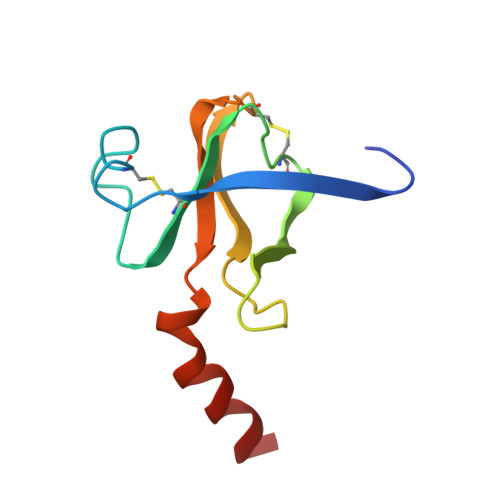
Structure and activity of two photoreversible cinnamates bound to chymotrypsin.
Stoddard, B.L., Bruhnke, J., Porter, N., Ringe, D., Petsko, G.A.(1990) Biochemistry 29: 4871-4879
- PubMed: 2364065
- DOI: https://doi.org/10.1021/bi00472a017
- Primary Citation of Related Structures:
3GCH, 4GCH - PubMed Abstract:
The serine protease gamma-chymotrypsin was covalently inhibited with two different photoreversible cinnamate compounds, and the structures of the resulting complexes were determined to 1.9-A resolution. The inhibitors show different kinetics of binding, inhibition, and nonphotochemical deacylation relative to each other in solution activity assays. The crystal structures of the enzyme-cinnamate complexes show that both compounds acylate serine 195 and that the two molecules are bound in similar nonproductive conformations which have drastic effects on their ability to turn over. Substitution of a diethylamino group on the para position of the cinnamate ring causes a 1000-fold increase in the thermal stability of the inhibitor toward hydrolysis and deacylation.
- Department of Chemistry, Massachusetts Institute of Technology, Cambridge 02139.
Organizational Affiliation: