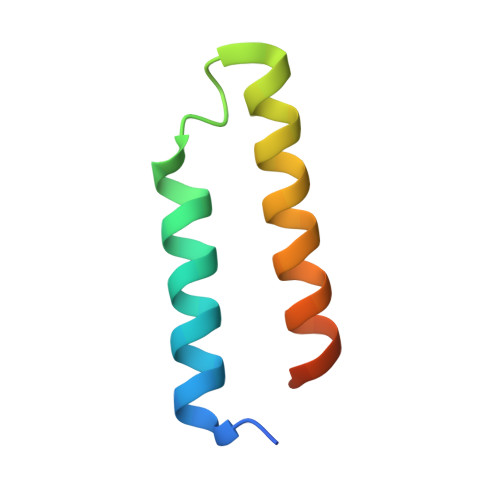
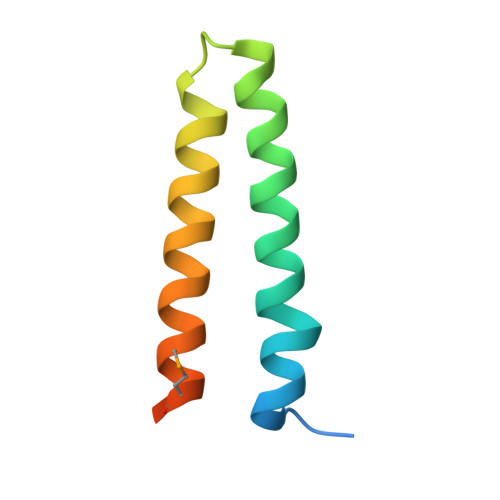
Structure and Function of Interacting IcmR-IcmQ Domains from a Type IVb Secretion System in Legionella pneumophila.
Raychaudhury, S., Farelli, J.D., Montminy, T.P., Matthews, M., Menetret, J.F., Dumenil, G., Roy, C.R., Head, J.F., Isberg, R.R., Akey, C.W.(2009) Structure 17: 590-601
- PubMed: 19368892
- DOI: https://doi.org/10.1016/j.str.2009.02.011
- Primary Citation of Related Structures:
3FXD, 3FXE - PubMed Abstract:
During infection, Legionella pneumophila creates a replication vacuole within eukaryotic cells and this requires a Type IVb secretion system (T4bSS). IcmQ plays a critical role in the translocase and associates with IcmR. In this paper, we show that the N-terminal domain of IcmQ (Qn) mediates self-dimerization, whereas the C-terminal domain with a basic linker promotes membrane association. In addition, the binding of IcmR to IcmQ prevents self-dimerization and also blocks membrane permeabilization. However, IcmR does not completely block membrane binding by IcmQ. We then determined crystal structures of Qn with the interacting region of IcmR. In this complex, each protein forms an alpha-helical hairpin within a parallel four-helix bundle. The amphipathic nature of helices in Qn suggests two possible models for membrane permeabilization by IcmQ. The Rm-Qn structure also suggests how IcmR-like proteins in other L. pneumophila species may interact with their IcmQ partners.
- Department of Physiology and Biophysics, Boston University School of Medicine, Boston, MA 02118-2526, USA.
Organizational Affiliation: