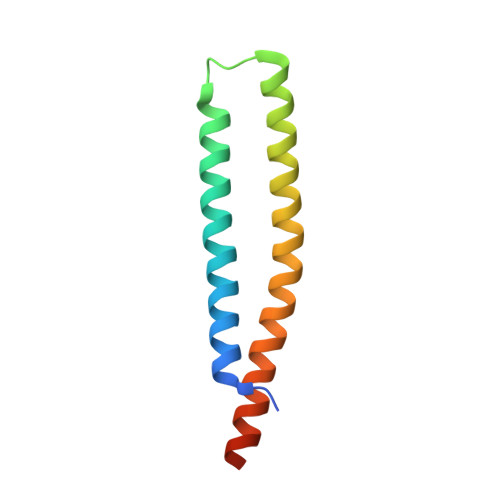
Crystal Structure of Hypothetical Protein HP0062 (O24902_HELPY) from Helicobacter pylori at 1.65 A Resolution.
Jang, S.B., Kwon, A.R., Son, W.S., Park, S.J., Lee, B.J.(2009) J Biochem
- PubMed: 19564156
- DOI: https://doi.org/10.1093/jb/mvp098
- Primary Citation of Related Structures:
3FX7 - PubMed Abstract:
The HP0062 gene encodes a small acidic protein of 86 amino acids with a theoretical pI of 4.6. The crystal structure of hypothetical protein HP0062 from Helicobacter pylori has been determined at 1.65 A by molecular-replacement method. The crystallographic asymmetric unit contains dimer, in which HP0062 monomer folds into a helix-hairpin-helix structure. The two protomers are primarily held together by extensive hydrophobic interactions in an antiparallel arrangement, forming a four helix bundle. Aromatic residues located at a or g position in the heptad leucine zipper are not major contributor required for HP0062 dimerization but important for the thermostability of this protein.
- Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, San 56-1, Shillim-Dong, Kwanak-Gu, Seoul 151-742, Korea.
Organizational Affiliation: