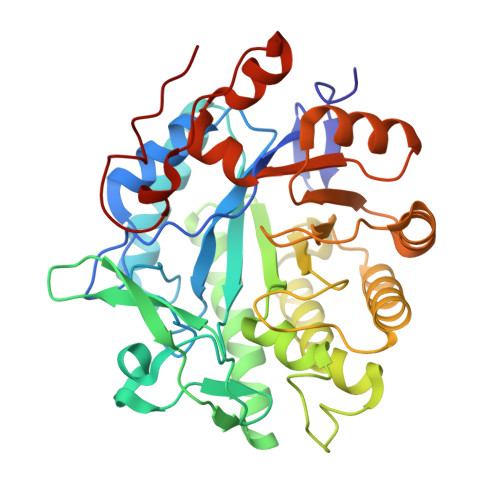
Structure-based insight into the asymmetric bioreduction of the C=C double bond of alpha,beta-unsaturated nitroalkenes by pentaerythritol tetranitrate reductase.
Toogood, H.S., Fryszkowska, A., Hare, V., Fisher, K., Roujeinikova, A., Leys, D., Gardiner, J.M., Stephens, G.M., Scrutton, N.S.To be published.