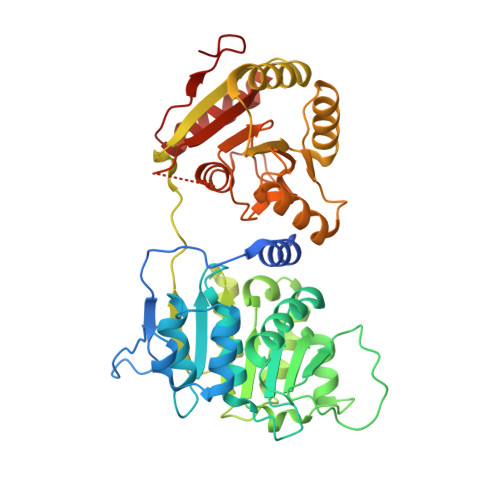
The DEXD/H-box RNA Helicase DDX19 Is Regulated by an {alpha}-Helical Switch.
Collins, R., Karlberg, T., Lehtio, L., Schutz, P., van den Berg, S., Dahlgren, L.G., Hammarstrom, M., Weigelt, J., Schuler, H.(2009) J Biological Chem 284: 10296-10300
- PubMed: 19244245
- DOI: https://doi.org/10.1074/jbc.C900018200
- Primary Citation of Related Structures:
3EWS, 3G0H - PubMed Abstract:
DEXD/H-box RNA helicases couple ATP hydrolysis to RNA remodeling by an unknown mechanism. We used x-ray crystallography and biochemical analysis of the human DEXD/H-box protein DDX19 to investigate its regulatory mechanism. The crystal structures of DDX19, in its RNA-bound prehydrolysis and free posthydrolysis state, reveal an alpha-helix that inserts between the conserved domains of the free protein to negatively regulate ATPase activity. This finding was corroborated by biochemical data that confirm an autoregulatory function of the N-terminal region of the protein. This is the first study describing crystal structures of a DEXD/H-box protein in its open and closed cleft conformations.
- Structural Genomics Consortium, Department of Medical Biochemistry and Biophysics, Karolinska Institute, S-17177 Stockholm, Sweden.
Organizational Affiliation: