A cytokine-neutralizing antibody as a structural mimetic of 2 receptor interactions
Wilkinson, T., Turner, R., Podichetty, S., Finch, D., McCourt, M., Loning, S., Jermutus, L.(2008) Proc Natl Acad Sci U S A 105: 20251-20256
- PubMed: 19073914
- DOI: https://doi.org/10.1073/pnas.0807200106
- Primary Citation of Related Structures:
3EO0, 3EO1 - PubMed Abstract:
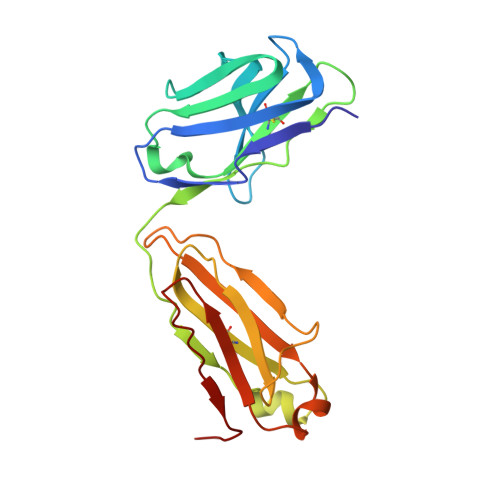
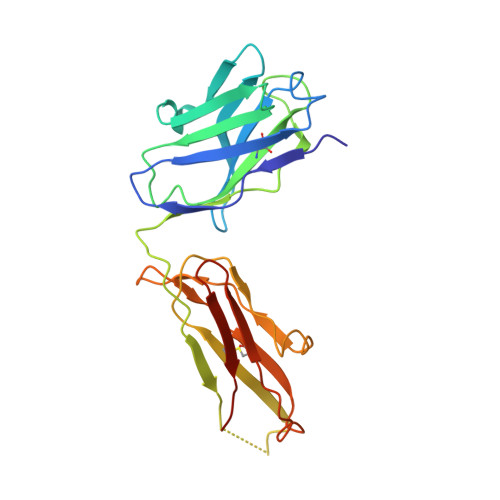
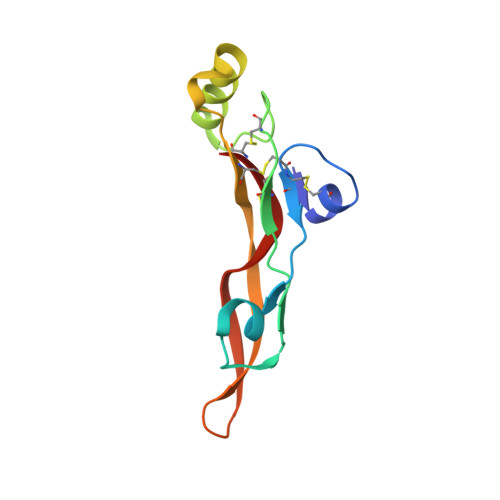
TGF-beta isoforms are key modulators of a broad range of biological pathways and increasingly are exploited as therapeutic targets. Here, we describe the crystal structures of a pan-TGF-beta neutralizing antibody, GC-1008, alone and in complex with TGF-beta3. The antibody is currently in clinical evaluation for idiopathic pulmonary fibrosis, melanoma, and renal cell cancer. GC-1008 recognizes an asymmetric binding interface across the TGF-beta homodimer with high affinity. Whereas both cognate receptors, TGF-beta-receptor types I and II, are required to recognize all 3 TGF-beta isoforms, GC-1008 has been engineered to bind with high affinity to TGF-beta1, 2, and 3 via a single interaction surface. Comparison with existing structures and models of TGF-beta interaction with its receptors suggests that the antibody binds to a similar epitope to the 2 receptors together and is therefore a structurally different but functionally identical mimic of the binding mode of both receptors.
- Biochemisches Institut, Universität Zürich, Winterthurerstrasse 190, 8057 Zurich, Switzerland.
Organizational Affiliation: