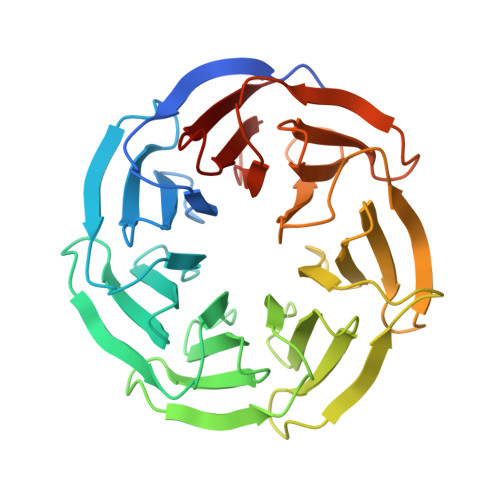
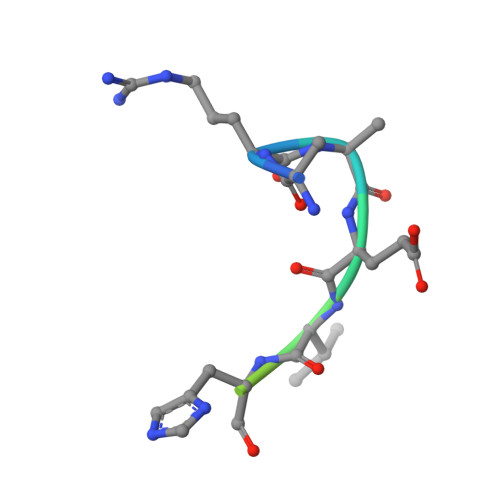
WDR5 Interacts with Mixed Lineage Leukemia (MLL) Protein via the Histone H3-binding Pocket.
Song, J.J., Kingston, R.E.(2008) J Biological Chem 283: 35258-35264
- PubMed: 18840606
- DOI: https://doi.org/10.1074/jbc.M806900200
- Primary Citation of Related Structures:
3EMH - PubMed Abstract:
WDR5 is a component of the mixed lineage leukemia (MLL) complex, which methylates lysine 4 of histone H3, and was identified as a methylated Lys-4 histone H3-binding protein. Here, we present a crystal structure of WDR5 bound to an MLL peptide. Surprisingly, we find that WDR5 utilizes the same pocket shown to bind histone H3 for this MLL interaction. Furthermore, the WDR5-MLL interaction is disrupted preferentially by mono- and di-methylated Lys-4 histone H3 over unmodified and tri-methylated Lys-4 histone H3. These data implicate a delicate interplay between the effector, WDR5, the catalytic subunit, MLL, and the substrate, histone H3, of the MLL complex. We suggest that the activity of the MLL complex might be regulated through this interplay.
- Department of Molecular Biology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts 02114, USA.
Organizational Affiliation: