Funded by the national institute of allergy and infectious diseases of nih (contract number hhsn272200700058c).
Singer, A.U., Skarina, T., Onopriyenko, O., Edwards, A.M., Anderson, W.F., Savchenko, A.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| yncA | 174 | N/A | Mutation(s): 0 Gene Names: yncA EC: 2.3.1 | 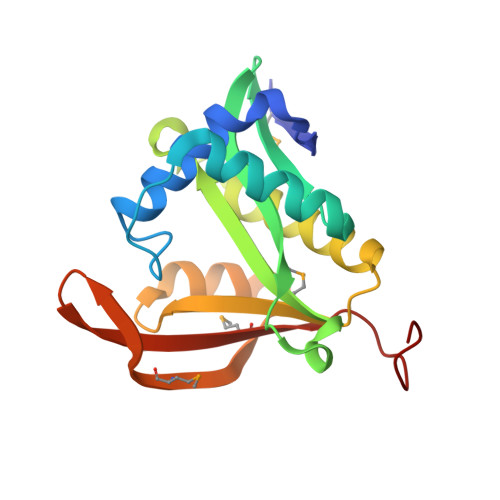 | |
UniProt | |||||
Find proteins for Q8ZPD3 (Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720)) Explore Q8ZPD3 Go to UniProtKB: Q8ZPD3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8ZPD3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GOL Query on GOL | J [auth C] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | D [auth A] E [auth A] F [auth A] G [auth B] H [auth B] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B, C | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 162.898 | α = 90 |
| b = 70.229 | β = 102.64 |
| c = 59.157 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| CrystalClear | data collection |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHASER | phasing |