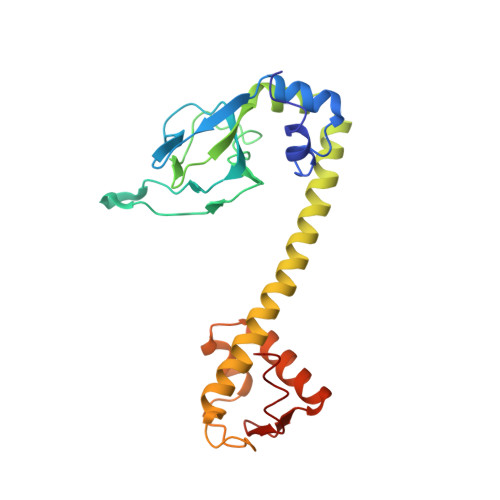
A dramatic conformational rearrangement is necessary for the activation of DNR from Pseudomonas aeruginosa. Crystal structure of wild-type DNR.
Giardina, G., Rinaldo, S., Castiglione, N., Caruso, M., Cutruzzola, F.(2009) Proteins
- PubMed: 19415759
- DOI: https://doi.org/10.1002/prot.22428
- Primary Citation of Related Structures:
3DKW - PubMed Abstract:
The opportunistic pathogen Pseudomonas aeruginosa can grow in low oxygen, because it is capable of anaerobic respiration using nitrate as a terminal electron acceptor (denitrification). An intermediate of the denitrification pathway is nitric oxide, a compound that may become cytotoxic at high concentration. The intracellular levels of nitric oxide are tightly controlled by regulating the expression of the enzymes responsible for its synthesis and degradation (nitrite and nitric oxide reductases). In this article, we present the crystallographic structure of the wild-type dissimilative nitrate respiration regulator (DNR), a master regulator controlling expression of the denitrification machinery and a putative target for new therapeutic strategies. Comparison with other structures among the CRP-FNR class of regulators reveals that DNR has crystallized in a conformation that has never been observed before. In particular, the sensing domain of DNR has undergone a rotation of more than 50 degrees with respect to the other structures. This suggests that DNR may undergo an unexpected and very large conformational rearrangement on activation.
- Department of Biochemical Sciences A. Rossi Fanelli, University of Rome La Sapienza, 00185 Rome, Italy.
Organizational Affiliation: