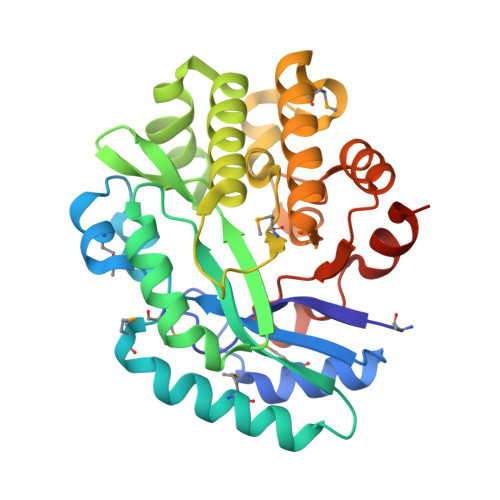
Crystal structure of the putative histidinol phosphatase hisK from Listeria monocytogenes.
Vorobiev, S.M., Su, M., Seetharaman, J., Zhao, L., Mao, L., Foote, E.L., Xiao, R., Nair, R., Baran, M.C., Acton, T.B., Rost, B., Montelione, G.T., Hunt, J.F., Tong, L.To be published.