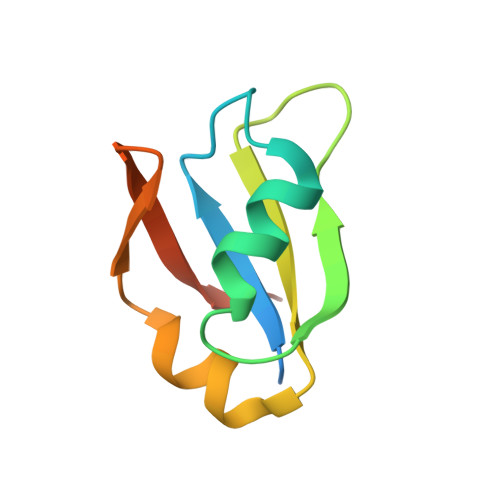
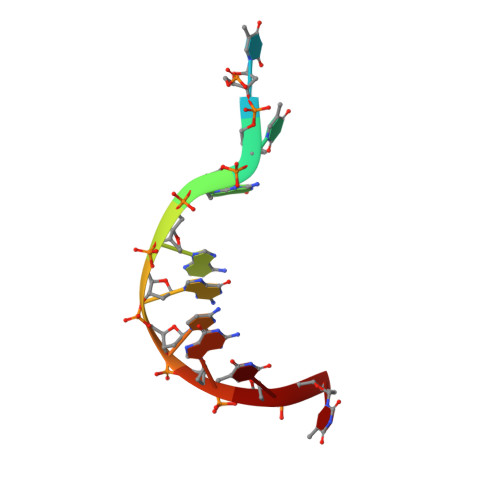
Structural insights into TDP-43 in nucleic-acid binding and domain interactions
Kuo, P.H., Doudeva, L.G., Wang, Y.T., Shen, C.K., Yuan, H.S.(2009) Nucleic Acids Res 37: 1799-1808
- PubMed: 19174564
- DOI: https://doi.org/10.1093/nar/gkp013
- Primary Citation of Related Structures:
3D2W - PubMed Abstract:
TDP-43 is a pathogenic protein: its normal function in binding to UG-rich RNA is related to cystic fibrosis, and inclusion of its C-terminal fragments in brain cells is directly linked to frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS). Here we report the 1.65 A crystal structure of the C-terminal RRM2 domain of TDP-43 in complex with a single-stranded DNA. We show that TDP-43 is a dimeric protein with two RRM domains, both involved in DNA and RNA binding. The crystal structure reveals the basis of TDP-43's TG/UG preference in nucleic acids binding. It also reveals that RRM2 domain has an atypical RRM-fold with an additional beta-strand involved in making protein-protein interactions. This self association of RRM2 domains produced thermal-stable RRM2 assemblies with a melting point greater than 85 degrees C as monitored by circular dichroism at physiological conditions. These studies thus characterize the recognition between TDP-43 and nucleic acids and the mode of RRM2 self association, and provide molecular models for understanding the role of TDP-43 in cystic fibrosis and the neurodegenerative diseases related to TDP-43 proteinopathy.
- Institute of Bioinformatics and Structural Biology, National Tsing Hua University, Taipei, Taiwan, ROC.
Organizational Affiliation: