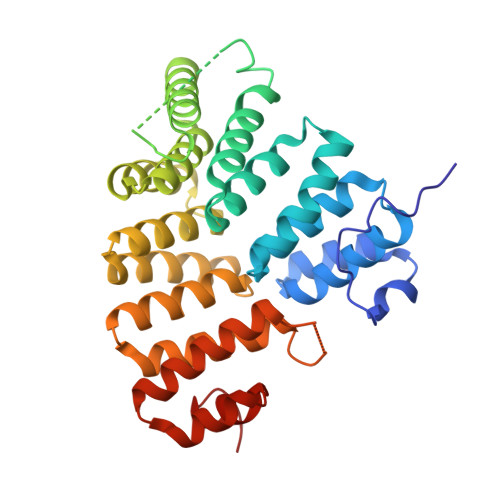
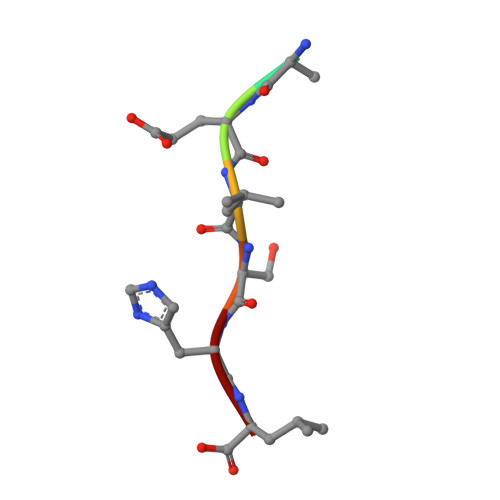
Structural Insights into the recognition of peroxisomal targeting signal 1 by Trypanosoma brucei peroxin 5.
Sampathkumar, P., Roach, C., Michels, P.A., Hol, W.G.(2008) J Mol Biology 381: 867-880
- PubMed: 18598704
- DOI: https://doi.org/10.1016/j.jmb.2008.05.089
- Primary Citation of Related Structures:
3CV0, 3CVL, 3CVN, 3CVP, 3CVQ - PubMed Abstract:
Glycosomes are peroxisome-like organelles essential for trypanosomatid parasites. Glycosome biogenesis is mediated by proteins called "peroxins," which are considered to be promising drug targets in pathogenic Trypanosomatidae. The first step during protein translocation across the glycosomal membrane of peroxisomal targeting signal 1 (PTS1)-harboring proteins is signal recognition by the cytosolic receptor peroxin 5 (PEX5). The C-terminal PTS1 motifs interact with the PTS1 binding domain (P1BD) of PEX5, which is made up of seven tetratricopeptide repeats. Obtaining diffraction-quality crystals of the P1BD of Trypanosoma brucei PEX5 (TbPEX5) required surface entropy reduction mutagenesis. Each of the seven tetratricopeptide repeats appears to have a residue in the alpha(L) conformation in the loop connecting helices A and B. Five crystal structures of the P1BD of TbPEX5 were determined, each in complex with a hepta- or decapeptide corresponding to a natural or nonnatural PTS1 sequence. The PTS1 peptides are bound between the two subdomains of the P1BD. These structures indicate precise recognition of the C-terminal Leu of the PTS1 motif and important interactions between the PTS1 peptide main chain and up to five invariant Asn side chains of PEX5. The TbPEX5 structures reported here reveal a unique hydrophobic pocket in the subdomain interface that might be explored to obtain compounds that prevent relative motions of the subdomains and interfere selectively with PTS1 motif binding or release in trypanosomatids, and would therefore disrupt glycosome biogenesis and prevent parasite growth.
- Department of Biochemistry, Biomolecular Structure Center, University of Washington, Box 357742, Seattle, WA 98195, USA.
Organizational Affiliation: