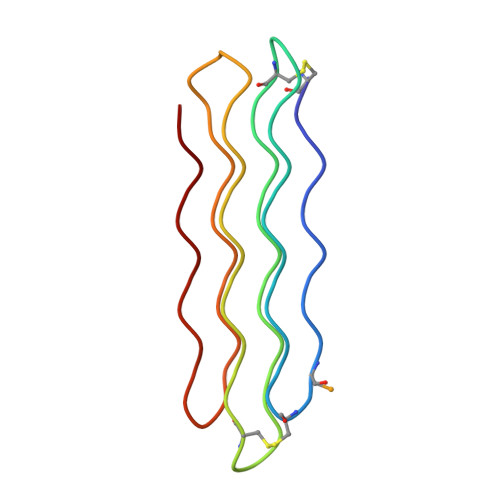
X-ray structure of snow flea antifreeze protein determined by racemic crystallization of synthetic protein enantiomers
Pentelute, B.L., Gates, Z.P., Tereshko, V., Dashnau, J.L., Vanderkooi, J.M., Kossiakoff, A.A., Kent, S.B.(2008) J Am Chem Soc 130: 9695-9701
- PubMed: 18598029
- DOI: https://doi.org/10.1021/ja8013538
- Primary Citation of Related Structures:
2PNE, 3BOG, 3BOI - PubMed Abstract:
Chemical protein synthesis and racemic protein crystallization were used to determine the X-ray structure of the snow flea antifreeze protein (sfAFP). Crystal formation from a racemic solution containing equal amounts of the chemically synthesized proteins d-sfAFP and l-sfAFP occurred much more readily than for l-sfAFP alone. More facile crystal formation also occurred from a quasi-racemic mixture of d-sfAFP and l-Se-sfAFP, a chemical protein analogue that contains an additional -SeCH2- moiety at one residue and thus differs slightly from the true enantiomer. Multiple wavelength anomalous dispersion (MAD) phasing from quasi-racemate crystals was then used to determine the X-ray structure of the sfAFP protein molecule. The resulting model was used to solve by molecular replacement the X-ray structure of l-sfAFP to a resolution of 0.98 A. The l-sfAFP molecule is made up of six antiparallel left-handed PPII helixes, stacked in two sets of three, to form a compact brick-like structure with one hydrophilic face and one hydrophobic face. This is a novel experimental protein structure and closely resembles a structural model proposed for sfAFP. These results illustrate the utility of total chemical synthesis combined with racemic crystallization and X-ray crystallography for determining the unknown structure of a protein.
- Institute for Biophysical Dynamics, University of Chicago, Chicago, Illinois 60637, USA.
Organizational Affiliation: