Defining a Protective Epitope on Factor H Binding Protein, a Key Meningococcal Virulence Factor and Vaccine Antigen.
Malito, E., Faleri, A., Lo Surdo, P., Veggi, D., Maruggi, G., Grassi, E., Cartocci, E., Bertoldi, I., Genovese, A., Santini, L., Romagnoli, G., Borgogni, E., Brier, S., Lo Passo, C., Domina, M., Castellino, F., Felici, F., Van Der Veen, S., Johnson, S., Lea, S.M., Tang, C.M., Pizza, M., Savino, S., Norais, N., Rappuoli, R., Bottomley, M.J., Masignani, V.(2013) Proc Natl Acad Sci U S A 110: 3304
- PubMed: 23396847
- DOI: https://doi.org/10.1073/pnas.1222845110
- Primary Citation of Related Structures:
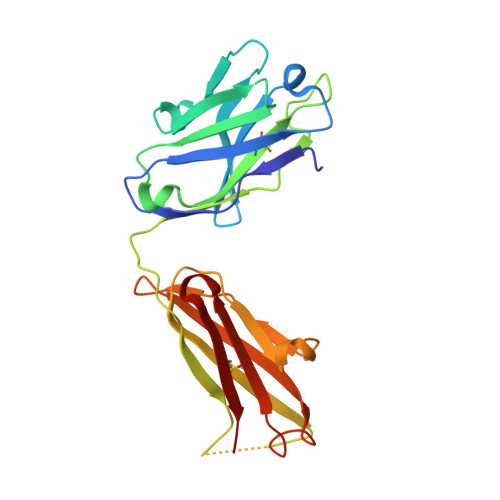
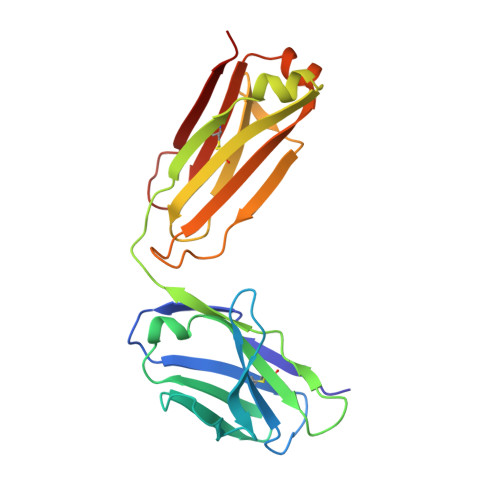
2YPV - PubMed Abstract:
Mapping of epitopes recognized by functional monoclonal antibodies (mAbs) is essential for understanding the nature of immune responses and designing improved vaccines, therapeutics, and diagnostics. In recent years, identification of B-cell epitopes targeted by neutralizing antibodies has facilitated the design of peptide-based vaccines against highly variable pathogens like HIV, respiratory syncytial virus, and Helicobacter pylori; however, none of these products has yet progressed into clinical stages. Linear epitopes identified by conventional mapping techniques only partially reflect the immunogenic properties of the epitope in its natural conformation, thus limiting the success of this approach. To investigate antigen-antibody interactions and assess the potential of the most common epitope mapping techniques, we generated a series of mAbs against factor H binding protein (fHbp), a key virulence factor and vaccine antigen of Neisseria meningitidis. The interaction of fHbp with the bactericidal mAb 12C1 was studied by various epitope mapping methods. Although a 12-residue epitope in the C terminus of fHbp was identified by both Peptide Scanning and Phage Display Library screening, other approaches, such as hydrogen/deuterium exchange mass spectrometry (MS) and X-ray crystallography, showed that mAb 12C1 occupies an area of ∼1,000 Å(2) on fHbp, including >20 fHbp residues distributed on both N- and C-terminal domains. Collectively, these data show that linear epitope mapping techniques provide useful but incomplete descriptions of B-cell epitopes, indicating that increased efforts to fully characterize antigen-antibody interfaces are required to understand and design effective immunogens.
- Research Center, Novartis Vaccines and Diagnostics srl, 53100 Siena, Italy.
Organizational Affiliation: