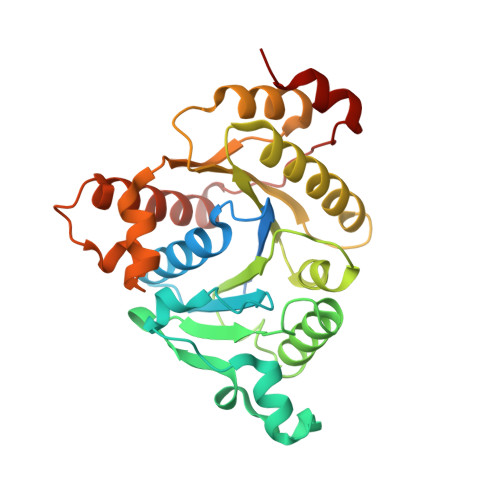
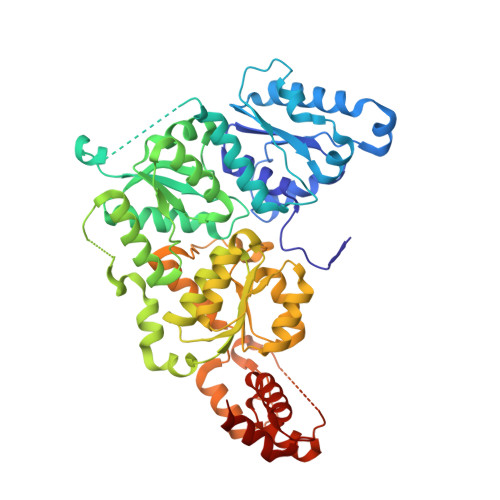
Structure of Adp-Aluminium Fluoride-Stabilized Protochlorophyllide Oxidoreductase Complex.
Moser, J., Lange, C., Krausze, J., Rebelein, J., Schubert, W., Ribbe, M.W., Heinz, D.W., Jahn, D.(2013) Proc Natl Acad Sci U S A 110: 2094
- PubMed: 23341615
- DOI: https://doi.org/10.1073/pnas.1218303110
- Primary Citation of Related Structures:
2YNM - PubMed Abstract:
Photosynthesis uses chlorophylls for the conversion of light into chemical energy, the driving force of life on Earth. During chlorophyll biosynthesis in photosynthetic bacteria, cyanobacteria, green algae and gymnosperms, dark-operative protochlorophyllide oxidoreductase (DPOR), a nitrogenase-like metalloenzyme, catalyzes the chemically challenging two-electron reduction of the fully conjugated ring system of protochlorophyllide a. The reduction of the C-17=C-18 double bond results in the characteristic ring architecture of all chlorophylls, thereby altering the absorption properties of the molecule and providing the basis for light-capturing and energy-transduction processes of photosynthesis. We report the X-ray crystallographic structure of the substrate-bound, ADP-aluminium fluoride-stabilized (ADP·AlF(3)-stabilized) transition state complex between the DPOR components L(2) and (NB)(2) from the marine cyanobacterium Prochlorococcus marinus. Our analysis permits a thorough investigation of the dynamic interplay between L(2) and (NB)(2). Upon complex formation, substantial ATP-dependent conformational rearrangements of L(2) trigger the protein-protein interactions with (NB)(2) as well as the electron transduction via redox-active [4Fe-4S] clusters. We also present the identification of artificial "small-molecule substrates" of DPOR in correlation with those of nitrogenase. The catalytic differences and similarities between DPOR and nitrogenase have broad implications for the energy transduction mechanism of related multiprotein complexes that are involved in the reduction of chemically stable double and/or triple bonds.
- Institut für Mikrobiologie, Technische Universität Braunschweig, D-38106 Braunschweig, Germany. j.moser@tu-bs.de
Organizational Affiliation: