Host Cell Invasion by Apicomplexan Parasites: Insights from the Co-Structure of Ama1 with a Ron2 Peptide
Tonkin, M.L., Roques, M., Lamarque, M.H., Pugniere, M., Douguet, D., Crawford, J., Lebrun, M., Boulanger, M.J.(2011) Science 333: 463
- PubMed: 21778402
- DOI: https://doi.org/10.1126/science.1204988
- Primary Citation of Related Structures:
2Y8R, 2Y8S, 2Y8T - PubMed Abstract:
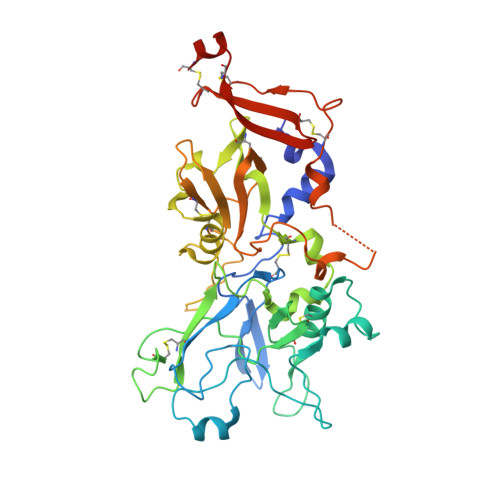
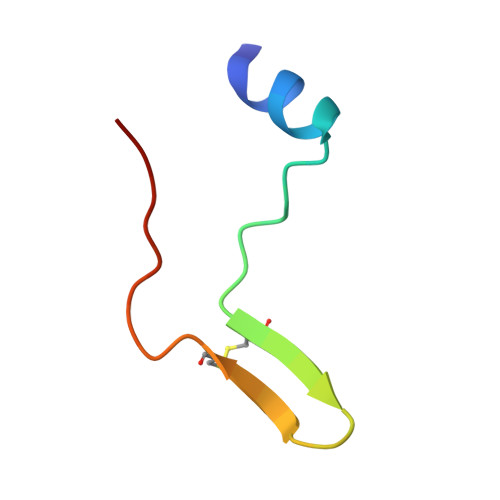
Apicomplexan parasites such as Toxoplasma gondii and Plasmodium species actively invade host cells through a moving junction (MJ) complex assembled at the parasite-host cell interface. MJ assembly is initiated by injection of parasite rhoptry neck proteins (RONs) into the host cell, where RON2 spans the membrane and functions as a receptor for apical membrane antigen 1 (AMA1) on the parasite. We have determined the structure of TgAMA1 complexed with a RON2 peptide at 1.95 angstrom resolution. A stepwise assembly mechanism results in an extensive buried surface area, enabling the MJ complex to resist the mechanical forces encountered during host cell invasion. Besides providing insights into host cell invasion by apicomplexan parasites, the structure offers a basis for designing therapeutics targeting these global pathogens.
- Department of Biochemistry and Microbiology, University of Victoria, Victoria, British Columbia V8W 3P6, Canada.
Organizational Affiliation: