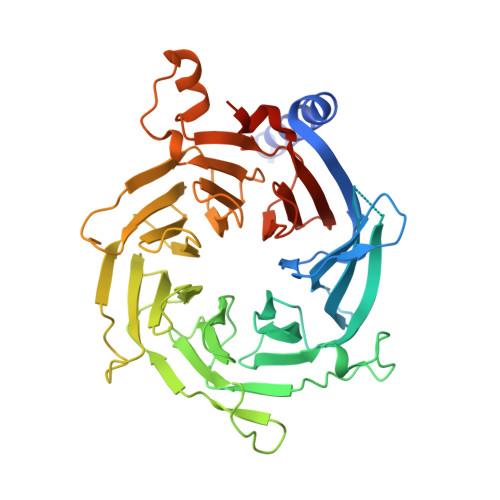
Chromatin-Modifying Complex Component Nurf55/P55 Associates with Histones H3, H4 and Polycomb Repressive Complex 2 Subunit Su(Z)12 Through Partially Overlapping Binding Sites.
Nowak, A.J., Alfieri, C., Stirnimann, C.U., Rybin, V., Baudin, F., Ly-Hartig, N., Lindner, D., Muller, C.W.(2011) J Biological Chem 286: 23388
- PubMed: 21550984
- DOI: https://doi.org/10.1074/jbc.M110.207407
- Primary Citation of Related Structures:
2XYI - PubMed Abstract:
Drosophila Nurf55 is a component of different chromatin-modifying complexes, including the PRC2 (Polycomb repressive complex 2). Based on the 1.75-Å crystal structure of Nurf55 bound to histone H4 helix 1, we analyzed interactions of Nurf55 (Nurf55 or p55 in fly and RbAp48/46 in human) with the N-terminal tail of histone H3, the first helix of histone H4, and an N-terminal fragment of the PRC2 subunit Su(z)12 using isothermal calorimetry and pulldown experiments. Site-directed mutagenesis identified the binding site of histone H3 at the top of the Nurf55 WD40 propeller. Unmodified or K9me3- or K27me3-containing H3 peptides were bound with similar affinities, whereas the affinity for K4me3-containing H3 peptides was reduced. Helix 1 of histone H4 and Su(z)12 bound to the edge of the β-propeller using overlapping binding sites. Our results show similarities in the recognition of histone H4 and Su(z)12 and identify Nurf55 as a versatile interactor that simultaneously contacts multiple partners.
- European Molecular Biology Laboratory, 69117 Heidelberg, Germany.
Organizational Affiliation: