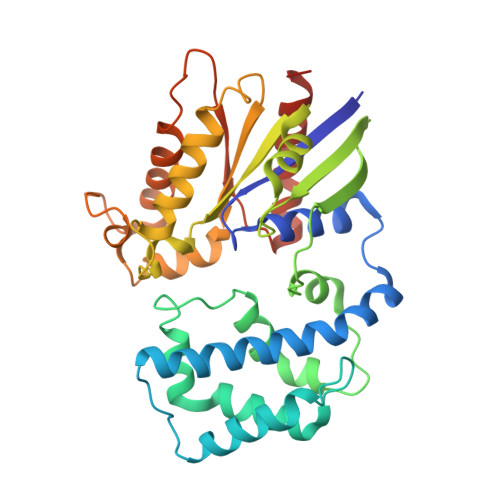
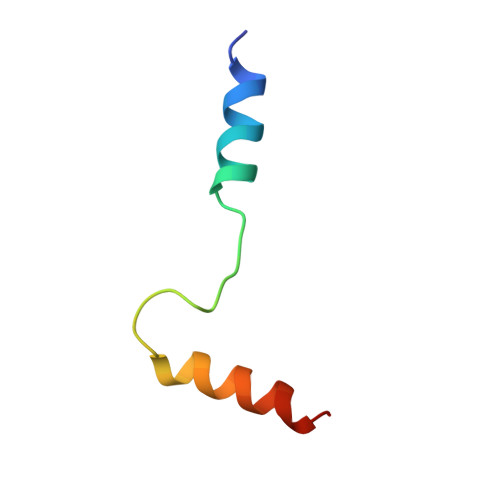
Computational Design of the Sequence and Structure of a Protein-Binding Peptide.
Sammond, D.W., Bosch, D.E., Butterfoss, G.L., Purbeck, C., Machius, M., Siderovski, D.P., Kuhlman, B.(2011) J Am Chem Soc 133: 4190
- PubMed: 21388199
- DOI: https://doi.org/10.1021/ja110296z
- Primary Citation of Related Structures:
2XNS - PubMed Abstract:
The de novo design of protein-binding peptides is challenging because it requires the identification of both a sequence and a backbone conformation favorable for binding. We used a computational strategy that iterates between structure and sequence optimization to redesign the C-terminal portion of the RGS14 GoLoco motif peptide so that it adopts a new conformation when bound to Gα(i1). An X-ray crystal structure of the redesigned complex closely matches the computational model, with a backbone root-mean-square deviation of 1.1 Å.
- Department of Biochemistry and Biophysics, University of North Carolina, Chapel Hill, North Carolina 27599-7260, USA.
Organizational Affiliation: