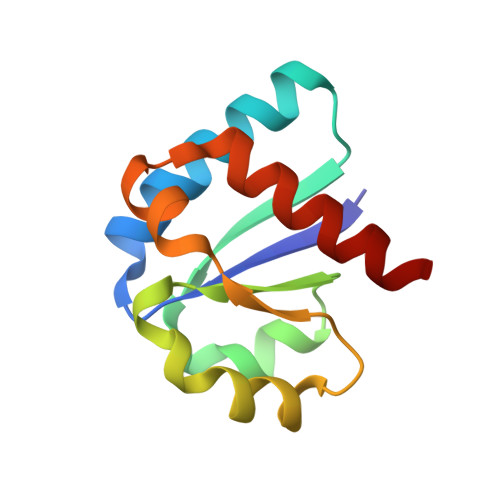
Solution Structure of the Iiachitobose-Iibchitobiose Complex of the N,N'-Diacetylchitobiose Branch of the Escherichia Coli Phosphotransfer System
Jung, Y.S., Cai, M., Clore, G.M.(2010) J Biological Chem 285: 4173
- PubMed: 19959833
- DOI: https://doi.org/10.1074/jbc.M109.080937
- Primary Citation of Related Structures:
2WWV, 2WY2 - PubMed Abstract:
The solution structure of the IIA-IIB complex of the N,N'-diacetylchitobiose (Chb) transporter of the Escherichia coli phosphotransferase system has been solved by NMR. The active site His-89 of IIA(Chb) was mutated to Glu to mimic the phosphorylated state and the active site Cys-10 of IIB(Chb) was substituted by serine to prevent intermolecular disulfide bond formation. Binding is weak with a K(D) of approximately 1.3 mm. The two complementary interaction surfaces are largely hydrophobic, with the protruding active site loop (residues 9-16) of IIB(Chb) buried deep within the active site cleft formed at the interface of two adjacent subunits of the IIA(Chb) trimer. The central hydrophobic portion of the interface is surrounded by a ring of polar and charged residues that provide a relatively small number of electrostatic intermolecular interactions that serve to correctly align the two proteins. The conformation of the active site loop in unphosphorylated IIB(Chb) is inconsistent with the formation of a phosphoryl transition state intermediate because of steric hindrance, especially from the methyl group of Ala-12 of IIB(Chb). Phosphorylation of IIB(Chb) is accompanied by a conformational change within the active site loop such that its path from residues 11-13 follows a mirror-like image relative to that in the unphosphorylated state. This involves a transition of the phi/psi angles of Gly-13 from the right to left alpha-helical region, as well as smaller changes in the backbone torsion angles of Ala-12 and Met-14. The resulting active site conformation is fully compatible with the formation of the His-89-P-Cys-10 phosphoryl transition state without necessitating any change in relative translation or orientation of the two proteins within the complex.
- From the Laboratory of Chemical Physics, NIDDK, National Institutes of Health, Bethesda, Maryland 20892.
Organizational Affiliation: