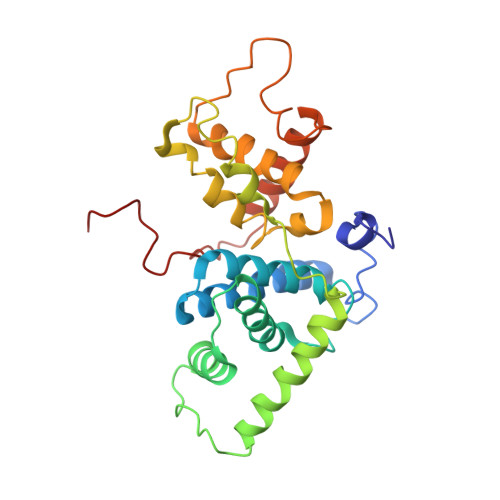
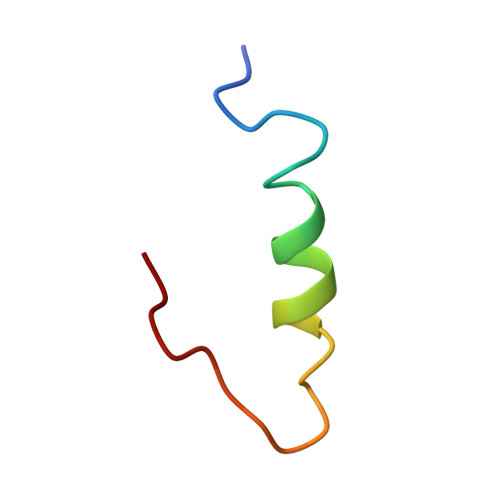
Structural Insights Into the Cyclin T1-Tat-Tar RNA Transcription Activation Complex from Eiav.
Anand, K., Schulte, A., Vogel-Bachmayr, K., Scheffzek, K., Geyer, M.(2008) Nat Struct Mol Biol 15: 1287
- PubMed: 19029897
- DOI: https://doi.org/10.1038/nsmb.1513
- Primary Citation of Related Structures:
2W2H - PubMed Abstract:
The replication of many retroviruses is mediated by a transcriptional activator protein, Tat, which activates RNA polymerase II at the level of transcription elongation. Tat interacts with Cyclin T1 of the positive transcription-elongation factor P-TEFb to recruit the transactivation-response TAR RNA, which acts as a promoter element in the transcribed 5' end of the viral long terminal repeat. Here we present the structure of the cyclin box domain of Cyclin T1 in complex with the Tat protein from the equine infectious anemia virus and its corresponding TAR RNA. The basic RNA-recognition motif of Tat adopts a helical structure whose flanking regions interact with a cyclin T-specific loop in the first cyclin box repeat. Together, both proteins coordinate the stem-loop structure of TAR. Our findings show that Tat binds to a surface on Cyclin T1 similar to where recognition motifs from substrate and inhibitor peptides were previously found to interact within Cdk-cyclin pairs.
- Max-Planck-Institut für molekulare Physiologie, Abteilung Physikalische Biochemie, Otto-Hahn-Strasse 11, 44227 Dortmund, Germany.
Organizational Affiliation: