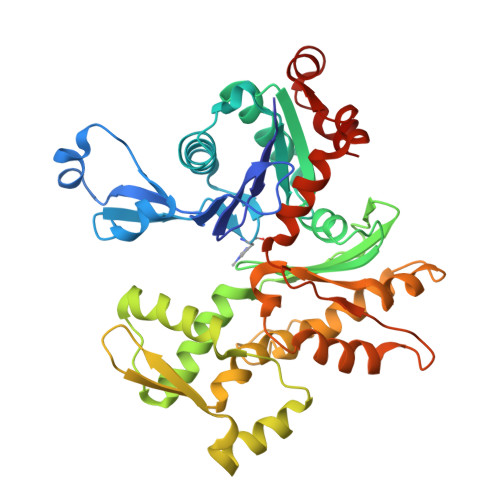
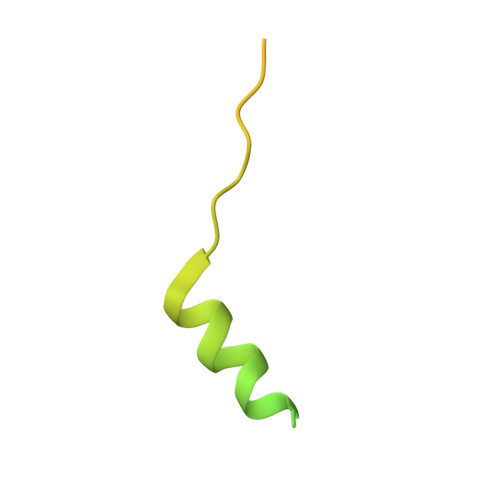
Interactions of isolated C-terminal fragments of neural Wiskott-Aldrich syndrome protein (N-WASP) with actin and Arp2/3 complex.
Gaucher, J.F., Mauge, C., Didry, D., Guichard, B., Renault, L., Carlier, M.F.(2012) J Biological Chem 287: 34646-34659
- PubMed: 22847007
- DOI: https://doi.org/10.1074/jbc.M112.394361
- Primary Citation of Related Structures:
2VCP - PubMed Abstract:
Wiskott-Aldrich syndrome proteins (WASP) are a family of proteins that all catalyze actin filament branching with the Arp2/3 complex in a variety of actin-based motile processes. The constitutively active C-terminal domain, called VCA, harbors one or more WASP homology 2 (WH2) domains that bind G-actin, whereas the CA extension binds the Arp2/3 complex. The VCA·actin·Arp2/3 entity associates with a mother filament to form a branched junction from which a daughter filament is initiated. The number and function of WH2-bound actin(s) in the branching process are not known, and the stoichiometry of the VCA·actin·Arp2/3 complex is debated. We have expressed the tandem WH2 repeats of N-WASP, either alone (V) or associated with the C (VC) and CA (VCA) extensions. We analyzed the structure of actin in complex with V, VC, and VCA using protein crystallography and hydrodynamic and spectrofluorimetric methods. The partial crystal structure of the VC·actin 1:1 complex shows two actins in the asymmetric unit with extensive actin-actin contacts. In solution, each of the two WH2 domains in V, VC, and VCA binds G-actin in 1:2 complexes that participate in barbed end assembly. V, VC, and VCA enhance barbed end depolymerization like profilin but neither nucleate nor sever filaments, in contrast with other WH2 repeats. VCA binds the Arp2/3 complex in a 1:1 complex even in the presence of a large excess of VCA. VCA·Arp2/3 binds one actin in a latrunculin A-sensitive fashion, in a 1:1:1 complex, indicating that binding of the second actin to VCA is weakened in the ternary complex.
- Laboratoire de Cristallographie et RMN Biologiques CNRS UMR 8015, Faculté de Pharmacie, Université Paris Descartes, Sorbonne Paris Cité, 4 avenue de l'Observatoire, 75006 Paris, France.
Organizational Affiliation: