Crystal structures of Medicago truncatula isoflavone O-methyltransferase reveal conserved surface binding motifs critical for enzymatic activity
Wang, C., Yu, X.-H., Liu, C.-J.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| O-methyltransferase | 357 | Medicago truncatula | Mutation(s): 0 Gene Names: Medicago truncatula IOMT3 gene EC: 2.1.1.46 (PDB Primary Data), 2.1.1.150 (UniProt) | 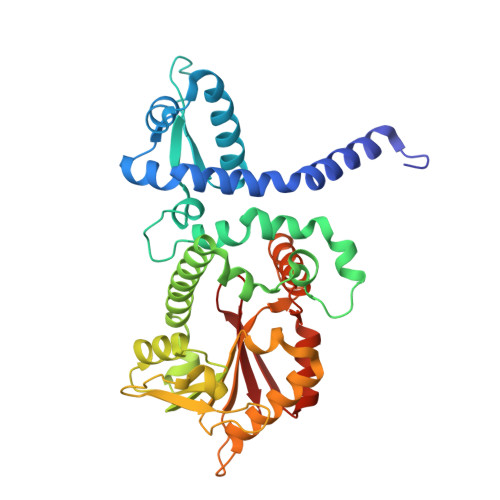 | |
UniProt | |||||
Find proteins for Q06YR3 (Medicago truncatula) Explore Q06YR3 Go to UniProtKB: Q06YR3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q06YR3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SAH Query on SAH | K [auth A], R [auth B] | S-ADENOSYL-L-HOMOCYSTEINE C14 H20 N6 O5 S ZJUKTBDSGOFHSH-WFMPWKQPSA-N |  | ||
| QSO Query on QSO | L [auth A], S [auth B] | 5,7-dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one C16 H12 O5 WUADCCWRTIWANL-UHFFFAOYSA-N |  | ||
| K Query on K | C [auth A] D [auth A] E [auth A] F [auth A] G [auth A] | POTASSIUM ION K NPYPAHLBTDXSSS-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 44.54 | α = 90 |
| b = 82.049 | β = 101.94 |
| c = 101.034 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| CNS | refinement |
| PDB_EXTRACT | data extraction |
| HKL-2000 | data collection |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| CNS | phasing |