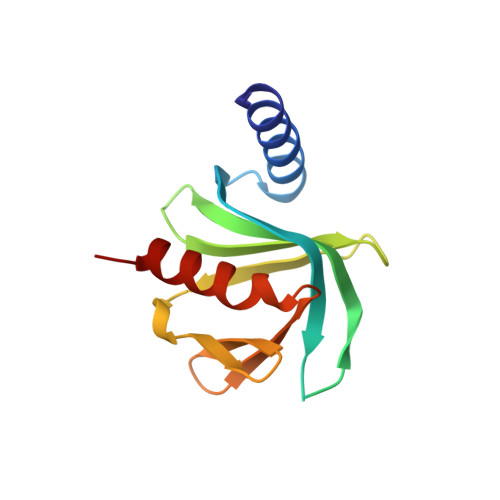
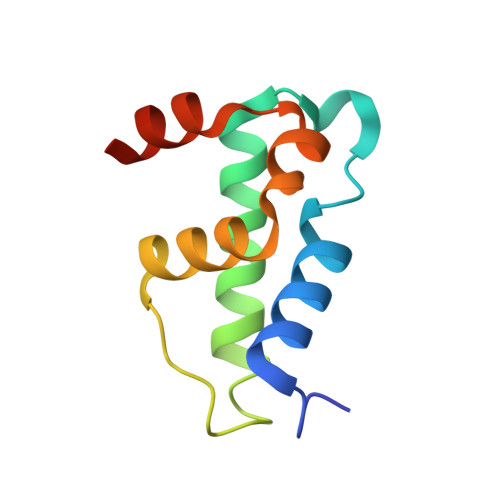
Structural basis of dcp2 recognition and activation by dcp1.
She, M., Decker, C.J., Svergun, D.I., Round, A., Chen, N., Muhlrad, D., Parker, R., Song, H.(2008) Mol Cell 29: 337-349
- PubMed: 18280239
- DOI: https://doi.org/10.1016/j.molcel.2008.01.002
- Primary Citation of Related Structures:
2QKL, 2QKM - PubMed Abstract:
A critical step in mRNA degradation is the removal of the 5' cap structure, which is catalyzed by the Dcp1-Dcp2 complex. The crystal structure of an S. pombe Dcp1p-Dcp2n complex combined with small-angle X-ray scattering analysis (SAXS) reveals that Dcp2p exists in open and closed conformations, with the closed complex being, or closely resembling, the catalytically more active form. This suggests that a conformational change between these open and closed complexes might control decapping. A bipartite RNA-binding channel containing the catalytic site and Box B motif is identified with a bound ATP located in the catalytic pocket in the closed complex, suggesting possible interactions that facilitate substrate binding. Dcp1 stimulates the activity of Dcp2 by promoting and/or stabilizing the closed complex. Notably, the interface of Dcp1 and Dcp2 is not fully conserved, explaining why the Dcp1-Dcp2 interaction in higher eukaryotes requires an additional factor.
- Laboratory of Macromolecular Structure, Institute of Molecular and Cell Biology, Agency for Science, Technology and Research, 61 Biopolis Drive, Proteos, 138673 Singapore.
Organizational Affiliation: