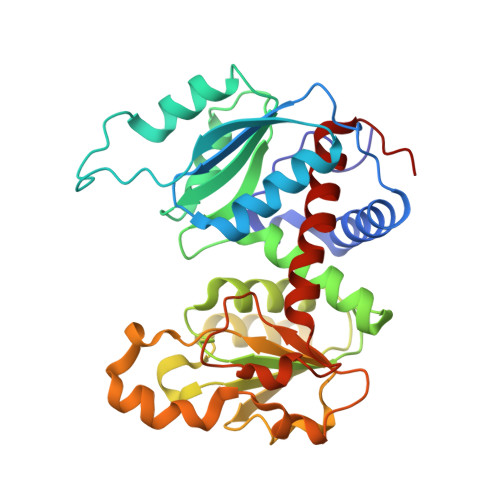
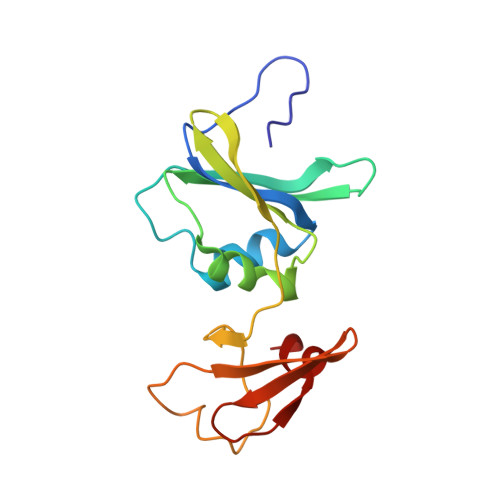
Comparison of two T-state structures of regulatory-chain mutants of Escherichia coli aspartate transcarbamoylase suggests that His20 and Asp19 modulate the response to heterotropic effectors.
Stec, B., Williams, M.K., Stieglitz, K.A., Kantrowitz, E.R.(2007) Acta Crystallogr D Biol Crystallogr 63: 1243-1253
- PubMed: 18084072
- DOI: https://doi.org/10.1107/S0907444907052985
- Primary Citation of Related Structures:
2QG9, 2QGF - PubMed Abstract:
Asp19 and His20 of Escherichia coli aspartate transcarbamoylase (EC 2.1.3.2) function in the binding of the triphosphate and ribose moieties of ATP and CTP and thereby may mediate important heterotropic regulation. The roles of these residues were investigated by individually mutating each of them to alanine and determining both the kinetic parameters and the structures of the mutant enzymes. The structures were determined by X-ray crystallography at 2.15 and 2.75 A resolution for His20Ar and Asp19Ar, respectively. Analysis was carried out on the unliganded T-state form. The structures of the mutants did not show gross structural divergence from the canonical T-state, but showed small and systematic differences that were analyzed by global conformational analysis. Structural analysis and comparison with other regulatory-chain mutants confirmed that the Asp19Ar mutant represents the stabilized T-state, while structural analysis of the His20Ar form indicated that it represents an equilibrium shifted towards the R-state. Global analysis of the Asp19Ar and His20Ar enzymes suggested a possible role as molecular modulators of the heterotropic effects caused by the binding of nucleotides at the regulatory site. These studies highlighted the structural determinants of T- or R-state stabilization. Additionally, application of the ;consensus modeling' methodology combined with high-resolution data allowed the determination of unclear structural features contributing to nucleotide specificity and the role of the N-termini of the regulatory chains.
- Burnham Institute for Medical Research, La Jolla, CA 92037, USA. bstec@burnham.org
Organizational Affiliation: