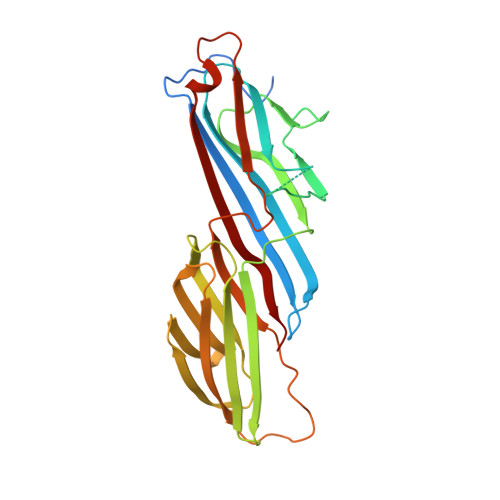
Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor gamma2 subunit.
Kittler, J.T., Chen, G., Kukhtina, V., Vahedi-Faridi, A., Gu, Z., Tretter, V., Smith, K.R., McAinsh, K., Arancibia-Carcamo, I.L., Saenger, W., Haucke, V., Yan, Z., Moss, S.J.(2008) Proc Natl Acad Sci U S A 105: 3616-3621
- PubMed: 18305175
- DOI: https://doi.org/10.1073/pnas.0707920105
- Primary Citation of Related Structures:
2PR9 - PubMed Abstract:
The regulation of the number of gamma2-subunit-containing GABA(A) receptors (GABA(A)Rs) present at synapses is critical for correct synaptic inhibition and animal behavior. This regulation occurs, in part, by the controlled removal of receptors from the membrane in clathrin-coated vesicles, but it remains unclear how clathrin recruitment to surface gamma2-subunit-containing GABA(A)Rs is regulated. Here, we identify a gamma2-subunit-specific Yxxvarphi-type-binding motif for the clathrin adaptor protein, AP2, which is located within a site for gamma2-subunit tyrosine phosphorylation. Blocking GABA(A)R-AP2 interactions via this motif increases synaptic responses within minutes. Crystallographic and biochemical studies reveal that phosphorylation of the Yxxvarphi motif inhibits AP2 binding, leading to increased surface receptor number. In addition, the crystal structure provides an explanation for the high affinity of this motif for AP2 and suggests that gamma2-subunit-containing heteromeric GABA(A)Rs may be internalized as dimers or multimers. These data define a mechanism for tyrosine kinase regulation of GABA(A)R surface levels and synaptic inhibition.
- Department of Physiology, University College London, Gower Street, London WC1E 6BT, United Kingdom. j.kittler@ucl.ac.uk
Organizational Affiliation: