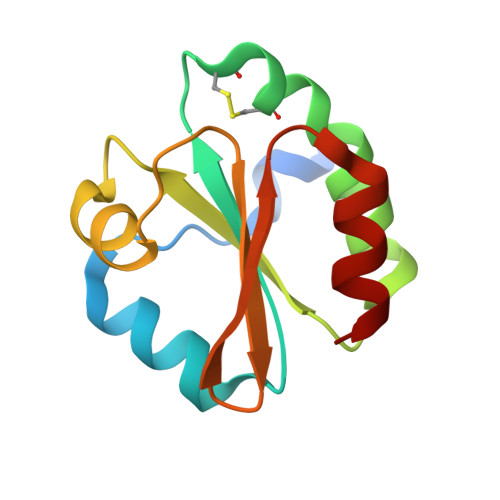
Structural and mechanistic analyses of yeast mitochondrial thioredoxin Trx3 reveal putative function of its additional cysteine residues
Bao, R., Zhang, Y.R., Zhou, C.-Z., Chen, Y.X.(2009) Biochim Biophys Acta 1794: 716-721
- PubMed: 19166985
- DOI: https://doi.org/10.1016/j.bbapap.2008.12.016
- Primary Citation of Related Structures:
2OE0, 2OE1, 2OE3 - PubMed Abstract:
The yeast Saccharomyces cerevisiae Trx3 is a key member of the thioredoxin system to control the cellular redox homeostasis in mitochondria. We solved the crystal structures of yeast Trx3 in oxidized and reduced forms at 1.80 and 2.10 A, respectively. Besides the active site, the additional cysteine residue Cys69 also undergoes a significant redox-correlated conformational change. Comparative structural analyses in combination with activity assays revealed that residue Cys69 could be S-nitrosylated in vitro. S-nitrosylation of Cys69 will decrease the activity of Trx3 by 20%, which is comparable to the effect of the Cys69Ser mutation. Taken together, these findings provided us some new insights into the putative function of the additional cysteine residues of Trx3.
- Institute of Protein Research, Tongji University, Shanghai 200092, PR China.
Organizational Affiliation: