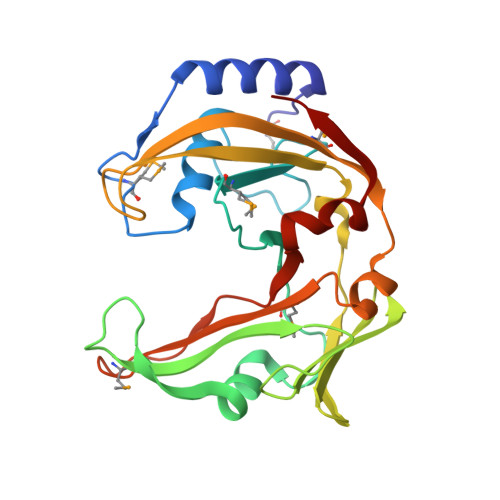
Monomeric restriction endonuclease BcnI in the apo form and in an asymmetric complex with target DNA.
Sokolowska, M., Kaus-Drobek, M., Czapinska, H., Tamulaitis, G., Szczepanowski, R.H., Urbanke, C., Siksnys, V., Bochtler, M.(2007) J Mol Biology 369: 722-734
- PubMed: 17445830
- DOI: https://doi.org/10.1016/j.jmb.2007.03.018
- Primary Citation of Related Structures:
2ODH, 2ODI, 2Q10 - PubMed Abstract:
Restriction endonuclease BcnI cleaves duplex DNA containing the sequence CC/SGG (S stands for C or G, / designates a cleavage position) to generate staggered products with single nucleotide 5'-overhangs. Here, we show that BcnI functions as a monomer that interacts with its target DNA in 1:1 molar ratio and report crystal structures of BcnI in the absence and in the presence of DNA. In the complex with DNA, BcnI makes specific contacts with all five bases of the target sequence and not just with a half-site, as the protomer of a typical dimeric restriction endonuclease. Our data are inconsistent with BcnI dimerization and suggest that the enzyme introduces double-strand breaks by sequentially nicking individual DNA strands, although this remains to be confirmed by kinetic experiments. BcnI is remotely similar to the DNA repair protein MutH and shares approximately 20% sequence identity with the restriction endonuclease MvaI, which is specific for the related sequence CC/WGG (W stands for A or T). As expected, BcnI is structurally similar to MvaI and recognizes conserved bases in the target sequence similarly but not identically. BcnI has a unique machinery for the recognition of the central base-pair.
- International Institute of Molecular and Cell Biology, Warsaw, Poland.
Organizational Affiliation: