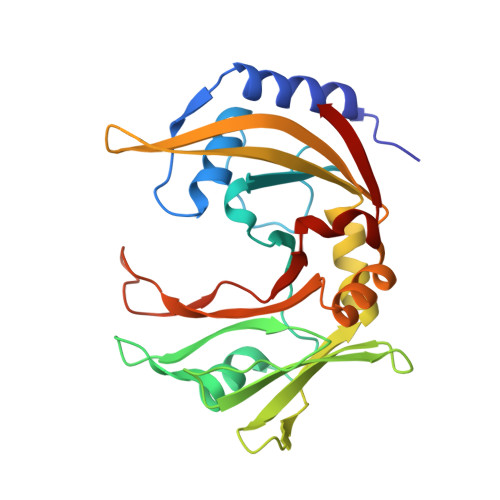
Restriction endonuclease MvaI is a monomer that recognizes its target sequence asymmetrically.
Kaus-Drobek, M., Czapinska, H., Sokolowska, M., Tamulaitis, G., Szczepanowski, R.H., Urbanke, C., Siksnys, V., Bochtler, M.(2007) Nucleic Acids Res 35: 2035-2046
- PubMed: 17344322
- DOI: https://doi.org/10.1093/nar/gkm064
- Primary Citation of Related Structures:
2OA9, 2OAA - PubMed Abstract:
Restriction endonuclease MvaI recognizes the sequence CC/WGG (W stands for A or T, '/' designates the cleavage site) and generates products with single nucleotide 5'-overhangs. The enzyme has been noted for its tolerance towards DNA modifications. Here, we report a biochemical characterization and crystal structures of MvaI in an apo-form and in a complex with target DNA at 1.5 A resolution. Our results show that MvaI is a monomer and recognizes its pseudosymmetric target sequence asymmetrically. The enzyme consists of two lobes. The catalytic lobe anchors the active site residues Glu36, Asp50, Glu55 and Lys57 and contacts the bases from the minor grove side. The recognition lobe mediates all major grove interactions with the bases. The enzyme in the crystal is bound to the strand with T at the center of the recognition sequence. The crystal structure with calcium ions and DNA mimics the prereactive state. MvaI shows structural similarities to BcnI, which cleaves the related sequence CC/SGG and to MutH enzyme, which is a component of the DNA repair machinery, and nicks one DNA strand instead of making a double-strand break.
- International Institute of Molecular and Cell Biology, Warsaw, Poland.
Organizational Affiliation: