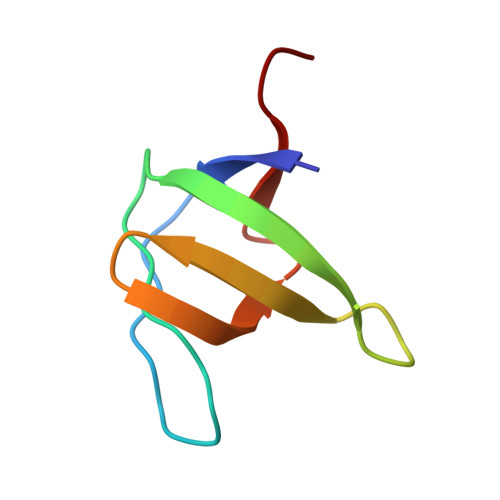
Crystallization by capillary counter-diffusion and structure determination of the N114A mutant of the SH3 domain of Abl tyrosine kinase complexed with a high-affinity peptide ligand.
Camara-Artigas, A., Palencia, A., Martinez, J.C., Luque, I., Gavira, J.A., Garcia-Ruiz, J.M.(2007) Acta Crystallogr D Biol Crystallogr 63: 646-652
- PubMed: 17452790
- DOI: https://doi.org/10.1107/S0907444907011109
- Primary Citation of Related Structures:
2O88 - PubMed Abstract:
The recognition of proline-rich ligands by SH3 domains is part of the process leading to diseases such as cancer or AIDS. Understanding the molecular determinants of the binding affinity and specificity of these interactions is crucial for the development of potent inhibitors with therapeutic potential. In this study, the crystallographic structure of the N114A mutant of the SH3 domain of the Abelson leukaemia virus tyrosine kinase complexed with a high-affinity peptide is presented. The crystallization was carried out using the capillary counter-diffusion technique, which facilitates the screening, manipulation and transport of the crystals and allows the collection of X-ray data directly from the capillary in which the crystals were grown. The crystals of the N114A mutant belong to the orthorhombic P2(1)2(1)2(1) space group, with unit-cell parameters a = 48.2, b = 50.1, c = 56.4 A. The quality of the diffraction data set has allowed the structure of the complex to be determined at a resolution limit of 1.75 A.
- Departamento de Química Física, Bioquímica y Química Inorgánica, Universidad de Almería, 04120 Almería, Spain.
Organizational Affiliation: