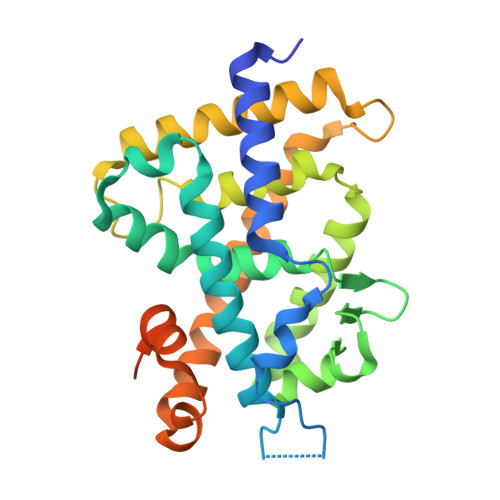
New analogs of 2-methylene-19-nor-(20S)-1,25-dihydroxyvitamin D(3) with conformationally restricted side chains: Evaluation of biological activity and structural determination of VDR-bound conformations.
Vanhooke, J.L., Tadi, B.P., Benning, M.M., Plum, L.A., Deluca, H.F.(2007) Arch Biochem Biophys 460: 161-165
- PubMed: 17227670
- DOI: https://doi.org/10.1016/j.abb.2006.11.029
- Primary Citation of Related Structures:
2O4J, 2O4R - PubMed Abstract:
We have successfully prepared E- and Z- isomers of 17-20 dehydro analogs of 2-methylene-19-nor-(20S)-1alpha,25-dihydroxyvitamin D3 (2MD). Both isomers bind to the recombinant rat vitamin D receptor (VDR) with high affinity. The Z-isomer (Vit-III 17-20Z) displays activity in vivo and in vitro that is similar to 2MD. The in vitro activity of the E-isomer (Vit-III 17-20E) is comparable to the natural hormone, though in vivo this analog is significantly less calcemic. Crystal structures of the rat VDR ligand binding domain complexed with the analogs demonstrate that the Vit-III 17-20Z analog is oriented almost identically to 2MD, with only minor differences induced by the planar configuration around the C17-C20 double bond. The Vit-III 17-20E analog is oriented in a conformation distinct from both 2MD and the natural hormone. The structural comparisons suggest that the position of C21 in the ligand binding site may be an important determinant of biological activity.
- Department of Biochemistry, University of Wisconsin-Madison, 433 Babcock Drive, Madison, WI 53706, USA. vanhooke@med.unc.edu
Organizational Affiliation: