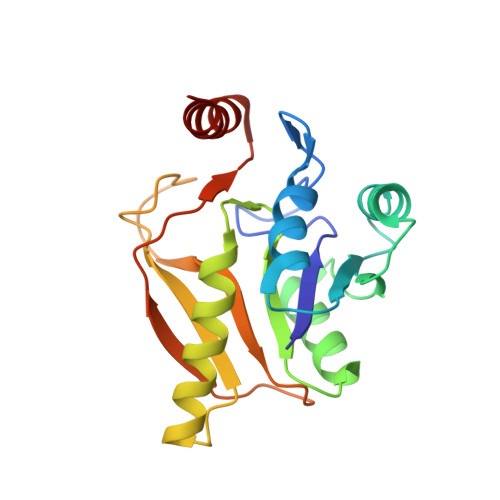
Solution Structural Studies of GTP:Adenosylcobinamide-Phosphateguanylyl Transferase (CobY) from Methanocaldococcus jannaschii.
Singarapu, K.K., Otte, M.M., Tonelli, M., Westler, W.M., Escalante-Semerena, J.C., Markley, J.L.(2015) PLoS One 10: e0141297-e0141297
- PubMed: 26513744
- DOI: https://doi.org/10.1371/journal.pone.0141297
- Primary Citation of Related Structures:
2MZB - PubMed Abstract:
GTP:adenosylcobinamide-phosphate (AdoCbi-P) guanylyl transferase (CobY) is an enzyme that transfers the GMP moiety of GTP to AdoCbi yielding AdoCbi-GDP in the late steps of the assembly of Ado-cobamides in archaea. The failure of repeated attempts to crystallize ligand-free (apo) CobY prompted us to explore its 3D structure by solution NMR spectroscopy. As reported here, the solution structure has a mixed α/β fold consisting of seven β-strands and five α-helices, which is very similar to a Rossmann fold. Titration of apo-CobY with GTP resulted in large changes in amide proton chemical shifts that indicated major structural perturbations upon complex formation. However, the CobY:GTP complex as followed by 1H-15N HSQC spectra was found to be unstable over time: GTP hydrolyzed and the protein converted slowly to a species with an NMR spectrum similar to that of apo-CobY. The variant CobYG153D, whose GTP complex was studied by X-ray crystallography, yielded NMR spectra similar to those of wild-type CobY in both its apo- state and in complex with GTP. The CobYG153D:GTP complex was also found to be unstable over time.
- National Magnetic Resonance Facility at Madison and Department of Biochemistry, University of Wisconsin-Madison, Madison, Wisconsin, United States of America; Center for NMR and Structural Chemistry, CSIR-Indian Institute of Chemical Technology, Tarnaka, Hyderabad, Telangana, India.
Organizational Affiliation: