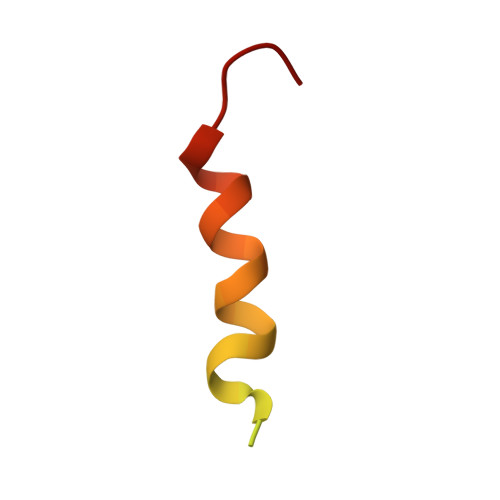
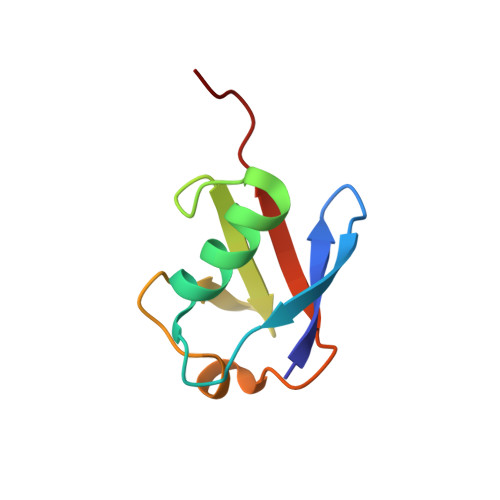
Structural Characterization of a Noncovalent Complex between Ubiquitin and the Transactivation Domain of the Erythroid-Specific Factor EKLF.
Raiola, L., Lussier-Price, M., Gagnon, D., Lafrance-Vanasse, J., Mascle, X., Arseneault, G., Legault, P., Archambault, J., Omichinski, J.G.(2013) Structure 21: 2014-2024
- PubMed: 24139988
- DOI: https://doi.org/10.1016/j.str.2013.08.027
- Primary Citation of Related Structures:
2MBH - PubMed Abstract:
Like other acidic transactivation domains (TAD), the minimal TAD from the erythroid-specific transcription factor EKLF (EKLFTAD) has been shown to contribute both to its transcriptional activity as well as to its ubiquitin(UBI)-mediated degradation. In this article, we examine the activation-degradation role of the acidic TAD of EKLF and demonstrate that the first 40 residues (EKLFTAD1) within this region form a noncovalent interaction with UBI. Nuclear magnetic resonance (NMR) structural studies of an EKLFTAD1-UBI complex show that EKLFTAD1 adopts a 14-residue α helix that forms the recognition interface with UBI in a similar manner as the UBI-interacting helix of Rabex5. We also identify a similar interaction between UBI and the activation-degradation region of SREBP1a, but not with the activation-degradation regions of p53, GAL4, and VP16. These results suggest that select activation-degradation regions like the ones found in EKLF and SREBP1a function in part through their ability to form noncovalent interactions with UBI.
- Département de Biochimie, Université de Montréal, C.P. 6128 Succursale Centre-Ville, Montréal, QC H3C 3J7, Canada.
Organizational Affiliation: