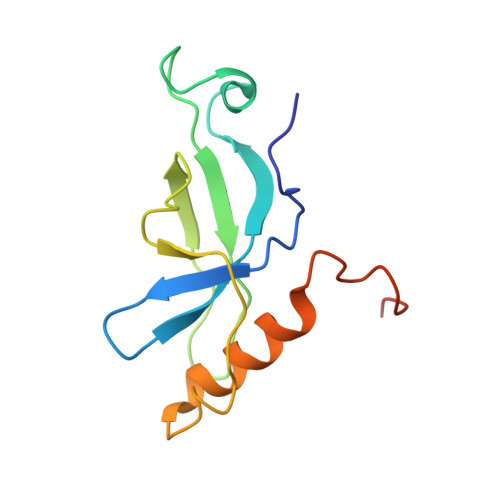
Solution structure of TbTFIIS2-1 PWWP domain from Trypanosoma brucei.
Wang, R., Zhang, J., Liao, S., Tu, X.(2016) Proteins 84: 912-919
- PubMed: 27005948
- DOI: https://doi.org/10.1002/prot.25035
- Primary Citation of Related Structures:
2M1H - PubMed Abstract:
TbTFIIS2-1, one of the two TFIIS homologues of Trypanosome brucei (T. brucei), cooperates with TbTFIIS1 in regulating transcription in T. brcuei. Structurally divergent from other TFIIS homologues from higher organisms, TbTFIIS2-1 contains an additional N-terminal PWWP domain besides other three conserved domains, which may imply potential role of TbTFIIS2-1 in transcription regulation. Here, we determined the solution structure of PWWP domain of TbTFIIS2-1 by NMR spectroscopy, which was the first solution structure of PWWP domain solved in trypanosomatid. In spite of poor sequence similarity between PWWP domains, this domain of TbTFIIS2-1 adopts a conserved 3D-structure, which contains a five-stranded β-barrel and a C-terminal α-helix. Furthermore, we found that TbTFIIS2-1 PWWP domain may be a protein-protein interaction module without the ability of DNA recognition and methyl-group interaction. Proteins 2016; 84:912-919. © 2016 Wiley Periodicals, Inc.
- Hefei National Laboratory for Physical Sciences at Microscale, and School of Life Sciences, University of Science and Technology of China, Hefei, Anhui, 230026, People's Republic of China.
Organizational Affiliation: