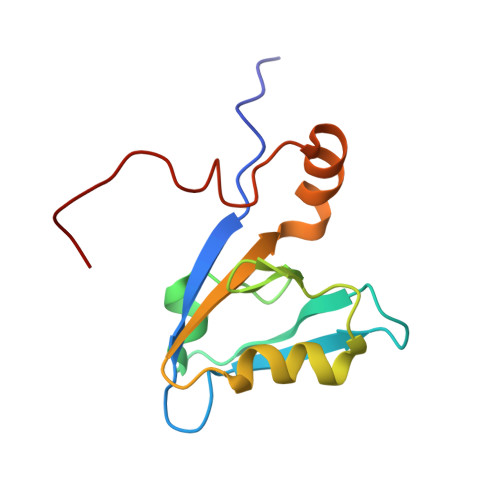
Ligand-Induced Dynamic Changes in Extended PDZ Domains from NHERF1.
Bhattacharya, S., Ju, J.H., Orlova, N., Khajeh, J.A., Cowburn, D., Bu, Z.(2013) J Mol Biology 425: 2509-2528
- PubMed: 23583913
- DOI: https://doi.org/10.1016/j.jmb.2013.04.001
- Primary Citation of Related Structures:
2M0T, 2M0U, 2M0V - PubMed Abstract:
The multi-domain scaffolding protein NHERF1 modulates the assembly and intracellular trafficking of various transmembrane receptors and ion-transport proteins. The two PDZ (postsynaptic density 95/disk large/zonula occluden 1) domains of NHERF1 possess very different ligand-binding capabilities: PDZ1 recognizes a variety of membrane proteins with high affinity, while PDZ2 only binds limited number of target proteins. Here using NMR, we have determined the structural and dynamic mechanisms that differentiate the binding affinities of the two PDZ domains, for the type 1 PDZ-binding motif (QDTRL) in the carboxyl terminus of cystic fibrosis transmembrane regulator. Similar to PDZ2, we have identified a helix-loop-helix subdomain coupled to the canonical PDZ1 domain. The extended PDZ1 domain is highly flexible with correlated backbone motions on fast and slow timescales, while the extended PDZ2 domain is relatively rigid. The malleability of the extended PDZ1 structure facilitates the transmission of conformational changes at the ligand-binding site to the remote helix-loop-helix extension. By contrast, ligand binding has only modest effects on the conformation and dynamics of the extended PDZ2 domain. The study shows that ligand-induced structural and dynamic changes coupled with sequence variation at the putative PDZ binding site dictate ligand selectivity and binding affinity of the two PDZ domains of NHERF1.
- New York Structural Biology Center, 89 Convent Avenue, New York, NY 10027, USA.
Organizational Affiliation: