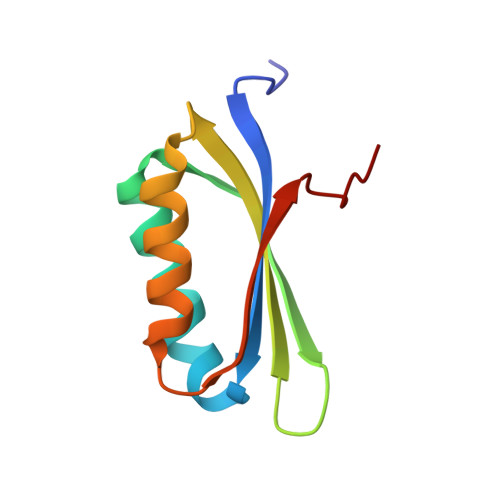
The C-Terminal Domain of the Virulence Factor MgtC Is a Divergent ACT Domain.
Yang, Y., Labesse, G., Carrere-Kremer, S., Esteves, K., Kremer, L., Cohen-Gonsaud, M., Blanc-Potard, A.B.(2012) J Bacteriol 194: 6255-6263
- PubMed: 22984256
- DOI: https://doi.org/10.1128/JB.01424-12
- Primary Citation of Related Structures:
2LQJ - PubMed Abstract:
MgtC is a virulence factor of unknown function important for survival inside macrophages in several intracellular bacterial pathogens, including Mycobacterium tuberculosis. It is also involved in adaptation to Mg(2+) deprivation, but previous work suggested that MgtC is not a Mg(2+) transporter. In this study, we demonstrated that the amount of the M. tuberculosis MgtC protein is not significantly increased by Mg(2+) deprivation. Members of the MgtC protein family share a conserved membrane N-terminal domain and a more divergent cytoplasmic C-terminal domain. To get insights into MgtC functional and structural organization, we have determined the nuclear magnetic resonance (NMR) structure of the C-terminal domain of M. tuberculosis MgtC. This structure is not affected by the Mg(2+) concentration, indicating that it does not bind Mg(2+). The structure of the C-terminal domain forms a βαββαβ fold found in small molecule binding domains called ACT domains. However, the M. tuberculosis MgtC ACT domain differs from canonical ACT domains because it appears to lack the ability to dimerize and to bind small molecules. We have shown, using a bacterial two-hybrid system, that the M. tuberculosis MgtC protein can dimerize and that the C-terminal domain somehow facilitates this dimerization. Taken together, these results indicate that M. tuberculosis MgtC does not have an intrinsic function related to Mg(2+) uptake or binding but could act as a regulatory factor based on protein-protein interaction that could be facilitated by its ACT domain.
- CNRS, UMR 5048, Université Montpellier 1 and Université Montpellier 2, Centre de Biochimie Structurale, Montpellier, France.
Organizational Affiliation: