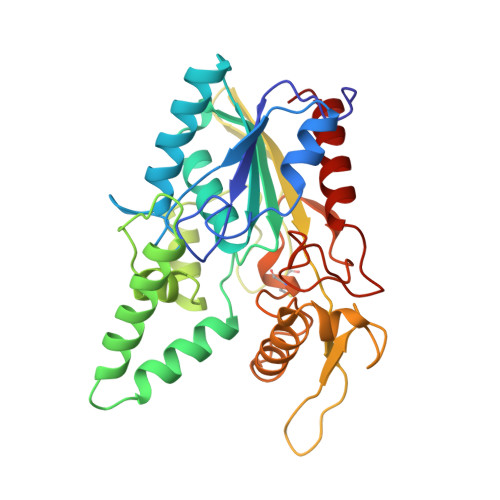
The open conformation of a Pseudomonas lipase.
Schrag, J.D., Li, Y., Cygler, M., Lang, D., Burgdorf, T., Hecht, H.J., Schmid, R., Schomburg, D., Rydel, T.J., Oliver, J.D., Strickland, L.C., Dunaway, C.M., Larson, S.B., Day, J., McPherson, A.(1997) Structure 5: 187-202
- PubMed: 9032074
- DOI: https://doi.org/10.1016/s0969-2126(97)00178-0
- Primary Citation of Related Structures:
2LIP, 3LIP - PubMed Abstract:
. The interfacial activation of lipases results primarily from conformational changes in the enzymes which expose the active site and provide a hydrophobic surface for interaction with the lipid substrate. Comparison of the crystallization conditions used and the structures observed for a variety of lipases suggests that the enzyme conformation is dependent on solution conditions. Pseudomonas cepacia lipase (PCL) was crystallized in conditions from which the open, active conformation of the enzyme was expected. Its three-dimensional structure was determined independently in three different laboratories and was compared with the previously reported closed conformations of the closely related lipases from Pseudomonas glumae (PGL) and Chromobacterium viscosum (CVL). These structures provide new insights into the function of this commercially important family of lipases. . The three independent structures of PCL superimpose with only small differences in the mainchain conformations. As expected, the observed conformation reveals a catalytic site exposed to the solvent. Superposition of PCL with the PGL and CVL structures indicates that the rearrangement from the closed to the open conformation involves three loops. The largest movement involves a 40 residue stretch, within which a helical segment moves to afford access to the catalytic site. A hydrophobic cleft that is presumed to be the lipid binding site is formed around the active site. . The interfacial activation of Pseudomonas lipases involves conformational rearrangements of surface loops and appears to conform to models of activation deduced from the structures of fungal and mammalian lipases. Factors controlling the conformational rearrangement are not understood, but a comparison of crystallization conditions and observed conformation suggests that the conformation of the protein is determined by the solution conditions, perhaps by the dielectric constant.
- Biotechnology Research Institute, NRC of Canada, 6100 Royalmount Ave. Montreal, Quebec H4P 2R2, Canada. Joe.Schrag@nrc.ca
Organizational Affiliation: