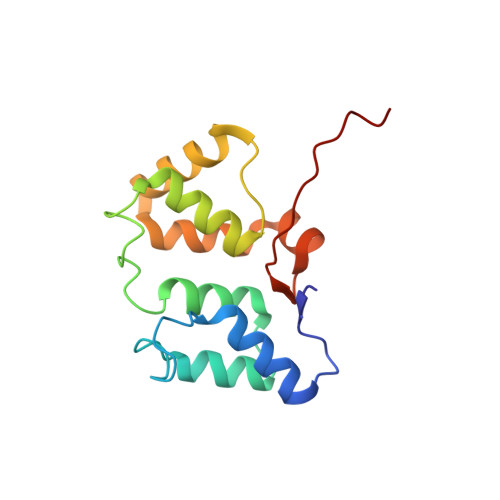
Solution NMR structure of the phycobilisome linker polypeptide domain of CpcC (20-153) from Thermosynechococcus elongatus, Northeast Structural Genomics Consortium Target TeR219A
Ramelot, T.A., Yang, Y., Cort, J.R., Lee, D., Ciccosanti, C., Hamilton, K., Acton, T.B., Xiao, R., Everett, J.K., Montelione, G.T., Kennedy, M.A.To be published.